J Korean Neurosurg Soc.
2015 May;57(5):335-341. 10.3340/jkns.2015.57.5.335.
The Neuroprotective Effect of Kefir on Spinal Cord Ischemia/Reperfusion Injury in Rats
- Affiliations
-
- 1Department of Neurosurgery, Canakkale Onsekiz Mart University, Faculty of Medicine, Canakkale, Turkey. drmustafaguven@comu.edu.tr
- 2Department of Cardiovascular Surgery, Canakkale Onsekiz Mart University, Faculty of Medicine, Canakkale, Turkey.
- 3Department of Medical Biochemistry, Canakkale Onsekiz Mart University, Faculty of Medicine, Canakkale, Turkey.
- 4Department of Histology and Embryology, Afyon Kocatepe University, Faculty of Medicine, Afyon, Turkey.
- KMID: 1881730
- DOI: http://doi.org/10.3340/jkns.2015.57.5.335
Abstract
OBJECTIVE
The main causes of spinal cord ischemia are a variety of vascular pathologies causing acute arterial occlusions. We investigated neuroprotective effects of kefir on spinal cord ischemia injury in rats.
METHODS
Rats were divided into three groups : 1) sham operated control rats; 2) spinal cord ischemia group fed on a standard diet without kefir pretreatment; and 3) spinal cord ischemia group fed on a standard diet plus kefir. Spinal cord ischemia was performed by the infrarenal aorta cross-clamping model. The spinal cord was removed after the procedure. The biochemical and histopathological changes were observed within the samples. Functional assessment was performed for neurological deficit scores.
RESULTS
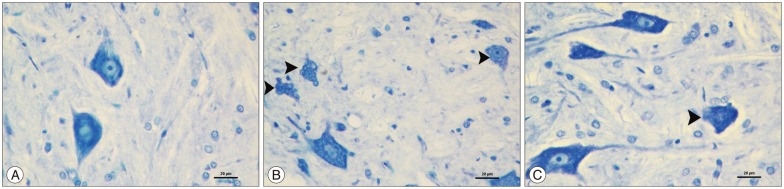
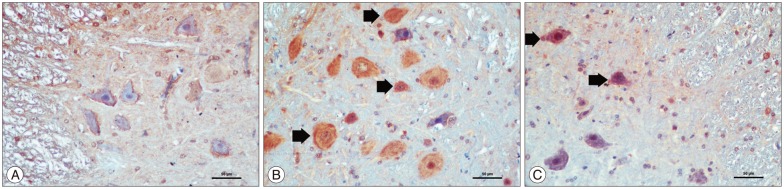
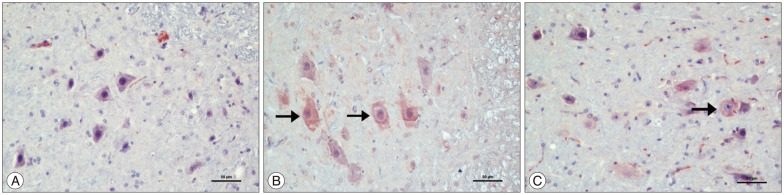
The kefir group was compared with the ischemia group, a significant decrease in malondialdehyde levels was observed (p<0.05). Catalase and superoxide dismutase levels of the kefir group were significantly higher than ischemia group (p<0.05). In histopathological samples, the kefir group is compared with ischemia group, there was a significant decrease in numbers of dead and degenerated neurons (p<0.05). In immunohistochemical staining, hipoxia-inducible factor-1alpha and caspase 3 immunopositive neurons were significantly decreased in kefir group compared with ischemia group (p<0.05). The neurological deficit scores of kefir group were significantly higher than ischemia group at 24 h (p<0.05).
CONCLUSION
Our study revealed that kefir pretreatment in spinal cord ischemia/reperfusion reduced oxidative stress and neuronal degeneration as a neuroprotective agent. Ultrastructural studies are required in order for kefir to be developed as a promising therapeutic agent to be utilized for human spinal cord ischemia in the future.
MeSH Terms
Figure
Reference
-
1. Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974; 48:137–145. PMID: 4141308.
Article2. Akman SA, Yagci RV. Prebiotics and probiotics. Cocuk Sagligi ve Hastaliklari Dergisi. 2002; 45:337–344.3. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. PMID: 7783230.
Article4. Berchner-Pfannschmidt U, Frede S, Wotzlaw C, Fandrey J. Imaging of the hypoxia-inducible factor pathway : insights into oxygen sensing. Eur Respir J. 2008; 32:210–217. PMID: 18591338.5. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302–310. PMID: 672633.6. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000; 190:255–266. PMID: 10685060.
Article7. Chen L, Endler A, Shibasaki F. Hypoxia and angiogenesis : regulation of hypoxia-inducible factors via novel binding factors. Exp Mol Med. 2009; 41:849–857. PMID: 19942820.
Article8. Chen TH, Wang SY, Chen KN, Liu JR, Chen MJ. Microbiological and chemical properties of kefir manufactured by entrapped microorganisms isolated from kefir grains. J Dairy Sci. 2009; 92:3002–3013. PMID: 19528577.
Article9. Ege E, Ilhan A, Gurel A, Akyol O, Ozen S. Erdosteine ameliorates neurological outcome and oxidative stress due to ischemia/reperfusion injury in rabbit spinal cord. Eur J Vasc Endovasc Surg. 2004; 28:379–386. PMID: 15350559.
Article10. Farnworth ER. Handbook of Fermented Functional Foods. ed 1. Boca Raton, FL, USA: CRC Press;2003. p. 59–75.11. Ginsberg MD. Review : Neuroprotection in brain ischemia : an update (part I ). Neuroscientist. 1995; 1:95–103.
Article12. Güven A, Güven A, Gülmez M. The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Vet Med B Infect Dis Vet Public Health. 2003; 50:412–416. PMID: 14633213.
Article13. Guzel-Seydim ZB, Kok-Tas T, Greene AK, Seydim AC. Review : functional properties of kefir. Crit Rev Food Sci Nutr. 2011; 51:261–268. PMID: 21390946.14. Hong WS, Chen YP, Chen MJ. The antiallergic effect of kefir Lactobacilli. J Food Sci. 2010; 75:H244–H253. PMID: 21535502.
Article15. Kanbak G, Uzuner K, Kuşat Ol K, Oğlakçı A, Kartkaya K, Şentürk H. Effect of kefir and low-dose aspirin on arterial blood pressure measurements and renal apoptosis in unhypertensive rats with 4 weeks salt diet. Clin Exp Hypertens. 2014; 36:1–8. PMID: 23631764.
Article16. Lafci G, Gedik HS, Korkmaz K, Erdem H, Cicek OF, Nacar OA, et al. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg. 2013; 8:64. PMID: 23557242.
Article17. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997; 91:479–489. PMID: 9390557.
Article18. Liu JR, Chen MJ, Lin CW. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agric Food Chem. 2005; 53:2467–2474. PMID: 15796581.
Article19. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID: 14907713.
Article20. Maeda H, Zhu X, Omura K, Suzuki S, Kitamura S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors. 2004; 22:197–200. PMID: 15630283.
Article21. Mainville I, Robert N, Lee B, Farnworth ER. Polyphasic characterization of the lactic acid bacteria in kefir. Syst Appl Microbiol. 2006; 29:59–68. PMID: 16423657.
Article22. Mauney MC, Blackbourne LH, Langenburg SE, Buchanan SA, Kron IL, Tribble CG. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Ann Thorac Surg. 1995; 59:245–252. PMID: 7818342.
Article23. Owaga EE, Chen MJ, Chen WY, Chen CW, Hsieh RH. Oral toxicity evaluation of kefir-isolated Lactobacillus kefiranofaciens M1 in Sprague-Dawley rats. Food Chem Toxicol. 2014; 70:157–162. PMID: 24842838.
Article24. Ozen OA, Cosar M, Sahin O, Fidan H, Eser O, Mollaoglu H, et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurol Sci. 2008; 29:147–152. PMID: 18612761.
Article25. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104. PMID: 10200555.
Article26. Punaro GR, Maciel FR, Rodrigues AM, Rogero MM, Bogsan CS, Oliveira MN, et al. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide. 2014; 37:53–60. PMID: 24406684.
Article27. Rodrigues KL, Caputo LR, Carvalho JC, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents. 2005; 25:404–408. PMID: 15848295.
Article28. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011; 365:537–547. PMID: 21830968.
Article29. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34:497–500. PMID: 3349599.
Article30. Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008; 15:621–627. PMID: 18259201.31. Yamauchi T, Sawa Y, Sakurai M, Hiroshi T, Matsumiya G, Abe K, et al. ONO-5046 attenuation of delayed motor neuron death and effect on the induction of brain-derived neurotrophic factor, phosphorylated extracellular signal-regulated kinase, and caspase3 after spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg. 2006; 131:644–650. PMID: 16515918.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuroprotective Effect and Inflammatory Gene Expression of Hypothermia and Lamotrigine in Transient Spinal Ischemia in the Rat
- In Vivo Neuroprotective Effect of Histidine-Tryptophan-Ketoglutarate Solution in an Ischemia/Reperfusion Spinal Cord Injury Animal Model
- The Effect of Intrathecal Bupivacaine with Hypothermia on Neuronal Protection against Transient Spinal Cord Ischemia in Rats
- Altered Glutamate Receptor Subtype mRNA Expressions after Transient Spinal Ischemia in the Rat
- Effects of Difference in Arterial Oxygen Tension on Ischemia/Reperfusion Injury of Spinal Cord in Rabbits