Obstet Gynecol Sci.
2013 Jan;56(1):29-35. 10.5468/OGS.2013.56.1.29.
Prognostic value of serum CA-125 in patients with advanced epithelial ovarian cancer followed by complete remission after adjuvant chemotherapy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Chonnam National University Medical School, Gwangju, Korea. seokmo2001@yahoo.co.kr
- KMID: 1857183
- DOI: http://doi.org/10.5468/OGS.2013.56.1.29
Abstract
OBJECTIVE
The aim of this study was to evaluate the prognostic value of serum CA-125 in advanced epithelial ovarian cancer with complete remission after primary adjuvant chemotherapy.
METHODS
We reviewed the records of 120 patients with advanced epithelial ovarian cancer who underwent primary surgery followed by adjuvant therapy at our institution between January 1998 and December 2005.
RESULTS
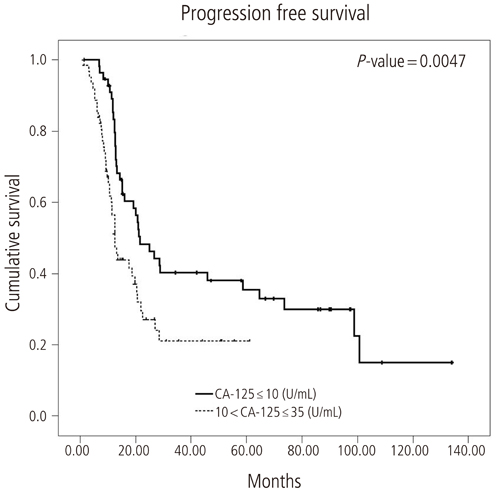
The median progression free survival was 21.6 months and 12.5 months in patients with nadir CA-125 levels < or =10 U/mL and 10 to 35 U/mL, respectively. Median overall survival in the same respective order was 130.2 months and 35.3 months. The level of serum CA-125 after the first cycle of adjuvant chemotherapy was most significantly higher in the recurrent group compared with the non-recurrent group. The optimal cut point of CA-125 on the receiver operating characteristic curve was 35 U/mL. Median progression free survival was 64.6 months and 12.8 months in patients with nadir CA-125 levels < or =35 U/mL and >35 U/mL, respectively, after first cycle of adjuvant chemotherapy.
CONCLUSION
Serum CA-125 level after the first cycle of adjuvant chemotherapy is a strong independent prognostic factor for advanced epithelial ovarian cancer with complete response.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010. 60:277–300.2. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO Committee on Gynecologic Oncology. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. Int J Gynaecol Obstet. 2000. 70:209–262.3. Kosary CL. Ries LA, Young JL, Keel GE, editors. Cancers of the ovary. SEER survival monograph: cancer survival among adults: US SEER program, 1988-2001, patient and tumor characteristics. 2007. Bethesda (MD): National Cancer Institute;133–144.4. Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002. 38:2435–2445.5. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009. 115:1234–1244.6. Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol. 1991. 9:1138–1150.7. Juretzka MM, Barakat RR, Chi DS, Iasonos A, Dupont J, Abu-Rustum NR, et al. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol. 2007. 104:176–180.8. Markman M, Liu PY, Rothenberg ML, Monk BJ, Brady M, Alberts DS. Pretreatment CA-125 and risk of relapse in advanced ovarian cancer. J Clin Oncol. 2006. 24:1454–1458.9. Liu PY, Alberts DS, Monk BJ, Brady M, Moon J, Markman M. An early signal of CA-125 progression for ovarian cancer patients receiving maintenance treatment after complete clinical response to primary therapy. J Clin Oncol. 2007. 25:3615–3620.10. Rocconi RP, Matthews KS, Kemper MK, Hoskins KE, Huh WK, Straughn JM Jr. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2009. 114:242–245.11. Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol. 2005. 16:47–50.12. Prat A, Parera M, Peralta S, Perez-Benavente MA, Garcia A, Gil-Moreno A, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008. 19:327–331.13. Markman M, Federico M, Liu PY, Hannigan E, Alberts D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol. 2006. 103:195–198.14. Pignata S, Perrone F, Di Maio M, Gallo C, De Placido S. In reply. J Clin Oncol. 2005. 23:2436–2437.15. Kang WD, Choi HS, Kim SM. Value of serum CA125 levels in patients with high-risk, early stage epithelial ovarian cancer. Gynecol Oncol. 2010. 116:57–60.16. Kang S, Seo SS, Park SY. Nadir CA-125 level is an independent prognostic factor in advanced epithelial ovarian cancer. J Surg Oncol. 2009. 100:244–247.17. Cooper BC, Sood AK, Davis CS, Ritchie JM, Sorosky JI, Anderson B, et al. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol. 2002. 100:59–64.18. Ivanov S. Epithelial ovarian cancer: preoperative assessment of Ca 125 levels as an independent prognostic factor. Akush Ginekol (Sofiia). 2003. 42:16–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Significance of Serum CA 125 Levels in Patients with Advanced Serous Epithelial Ovarian Cancer
- Prognostic Value of Serum CA 125 Measurment during Chemotherapy for the Patients with Epithelial Ovarian Cancer
- Use of CA 125 Antigen to Predict Suvival of Patients with Epithelial Ovarian Cancer
- Clinical Relevance of the CA 125 Assay in Monitoring of Epithelial Ovarian Cancer Patients
- CA-125 cut-off value as a predictor for complete interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer