Obstet Gynecol Sci.
2013 Jan;56(1):15-21. 10.5468/OGS.2013.56.1.15.
Promising treatment results of adjuvant chemotherapy following radical hysterectomy for intermediate risk stage 1B cervical cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kosin University College of Medicine, Busan, Korea. kwg@ns.kosinmed.or.kr
- KMID: 1857181
- DOI: http://doi.org/10.5468/OGS.2013.56.1.15
Abstract
OBJECTIVE
The aim of this retrospective study is to evaluate the efficacy of adjuvant chemotherapy following radical hysterectomy for intermediate risk stage IB cervical cancer.
METHODS
From January 1993 to December 2007, a total of 100 patients of stage IB were enrolled in this study who had at least two of the following three intermediate risk factors (deep stromal invasion, lymphovascular space involvement, and large tumor size) after radical hysterectomy and all patients had no high risk factors and no radiotherapy. Of these patients, 22 patients had surgery only and 78 patients had cisplatin-based combination chemotherapy as adjuvant therapy postoperatively to improve survival. Kaplan-Meier survival curves and Cox's proportional-hazards regression model and log-rank test were used for survival analysis and to estimate the impact of prognostic factors on survival.
RESULTS
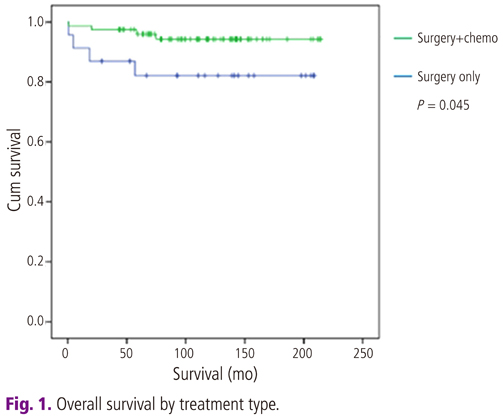
The mean age was 52 years (range, 28 to 76 years). The overall survival rate of all intermediate tumors are 92% (92/100). Surgery only group is 81.8% (18/22) and adjuvant chemotherapy group is 94.9% (74/78). Comparison of survival between two groups revealed significant statistical difference in both univariant and multivariant survival analysis (P<0.05). The main toxicities of adjuvant chemotherapy were bone marrow suppression (18%), nausea and vomiting (5.2%) and alopecia in etoposide-cisplatin chemotherapy group (100%) but most side effects of postoperative adjuvant chemotherapy were transient, reversible and within acceptable limits to all patients.
CONCLUSION
Cisplatin based combined adjuvant chemotherapy for intermediate risk tumors after radical hysterectomy is promising with significant improvement of overall survival and with acceptable toxicity profile.
MeSH Terms
Figure
Cited by 2 articles
-
Surgical Outcomes of Robotic Radical Hysterectomy Using Three Robotic Arms versus Conventional Multiport Laparoscopy in Patients with Cervical Cancer
Ga Won Yim, Sang Wun Kim, Eun Ji Nam, Sunghoon Kim, Hee Jung Kim, Young Tae Kim
Yonsei Med J. 2014;55(5):1222-1230. doi: 10.3349/ymj.2014.55.5.1222.Oncologic outcomes of adjuvant chemotherapy alone after radical surgery for stage IB–IIA cervical cancer patients
Kwang-Beom Lee, Young Saing Kim, Jong-Min Lee
J Gynecol Oncol. 2018;29(1):. doi: 10.3802/jgo.2018.29.e5.
Reference
-
1. Hacker NF, Friedlander ML. Berek JS, Hacker NF, editors. Cervical cancer. Berek & Hacker's gynecologic oncology. 2010. 5th ed. Philadelphia (PA): Lippincott Williams & Wilkins;341–395.2. Bidus MA, Elcas JC. Berek JS, Novak E, editors. Cervical and vaginal cancer. Berek & Novak's gynecology. 2007. 14th ed. Philadelphia (PA): Lippincott Williams & Wilkins;1403–1456.3. Boyce J, Fruchter RG, Nicastri AD, Ambiavagar PC, Reinis MS, Nelson JH Jr. Prognostic factors in stage I carcinoma of the cervix. Gynecol Oncol. 1981. 12:154–165.4. Delgado G, Bundy BN, Fowler WC Jr, Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989. 35:314–320.5. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990. 38:352–357.6. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999. 340:1137–1143.7. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000. 18:1606–1613.8. Ryu HS. Concurrent chemoradiotherapy in cervical cancer (a new paradigm in cervical cancer treatment). Yonsei Med J. 2002. 43:749–753.9. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999. 73:177–183.10. Soisson AP, Soper JT, Clarke-Pearson DL, Berchuck A, Montana G, Creasman WT. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990. 37:390–395.11. Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006. 65:169–176.12. Iwasaka T, Kamura T, Yokoyama M, Matsuo N, Nakano H, Sugimori H. Adjuvant chemotherapy after radical hysterectomy for cervical carcinoma: a comparison with effects of adjuvant radiotherapy. Obstet Gynecol. 1998. 91:977–981.13. Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 2006. 103:618–622.14. Tattersall MH, Ramirez C, Coppleson M. A randomized trial of adjuvant chemotherapy after radical hysterectomy in stage Ib-IIa cervical cancer patients with pelvic lymph node metastases. Gynecol Oncol. 1992. 46:176–181.15. Alberts DS, Garcia D, Mason-Liddil N. Cisplatin in advanced cancer of the cervix: an update. Semin Oncol. 1991. 18:11–24.16. Runowicz CD, Wadler S, Rodriguez-Rodriguez L, Litwin P, Shaves M, O'Hanlan KA, et al. Concomitant cisplatin and radiotherapy in locally advanced cervical carcinoma. Gynecol Oncol. 1989. 34:395–401.17. Al-Saleh E, Hoskins PJ, Pike JA, Swenerton KD. Cisplatin/etoposide chemotherapy for recurrent or primarily advanced cervical carcinoma. Gynecol Oncol. 1997. 64:468–472.18. Olive ST, Kiser WR. Diagnosis of appendicitis. J Am Board Fam Pract. 1996. 9:306–307.19. Bonomi P, Blessing J, Ball H, Hanjani P, DiSaia PJ. A phase II evaluation of cisplatin and 5-fluorouracil in patients with advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989. 34:357–359.20. Namkoong SE, Park JS, Kim JW, Bae SN, Han GT, Lee JM, et al. Comparative study of the patients with locally advanced stages I and II cervical cancer treated by radical surgery with and without preoperative adjuvant chemotherapy. Gynecol Oncol. 1995. 59:136–142.21. Alberts DS, Kronmal R, Baker LH, Stock-Novack DL, Surwit EA, Boutselis JG, et al. Phase II randomized trial of cisplatin chemotherapy regimens in the treatment of recurrent or metastatic squamous cell cancer of the cervix: a Southwest Oncology Group Study. J Clin Oncol. 1987. 5:1791–1795.22. Dische S. Radiotherapy of cervical cancer. Clin Obstet Gynaecol. 1985. 12:203–227.23. Barter JF, Soong SJ, Shingleton HM, Hatch KD, Orr JW Jr. Complications of combined radical hysterectomy-postoperative radiation therapy in women with early stage cervical cancer. Gynecol Oncol. 1989. 32:292–296.24. Markmam M. Berek JS, Hacker NF, editors. Chemotherapy. Berek & Hacker's gynecologic oncology. 2010. 5th ed. Philadelphia (PA): Lippincott Williams & Wilkins;57–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaginal evisceration after radical hysterectomy and adjuvant radiation
- Neoadjuvant and postoperative chemotherapy with paclitaxel plus cisplatin for the treatment of FIGO stage IB cervical cancer in pregnancy
- Comparison of postoperative adjuvant platinum-based chemotherapy and no further therapy after radical surgery in intermediate-risk early-stage cervical cancer
- Recent Management of FIGO stage IB2 Cervical Cancer
- Chemoradiotherapy is not superior to radiotherapy alone after radical surgery for cervical cancer patients with intermediate-risk factor