Obstet Gynecol Sci.
2014 Jul;57(4):266-273. 10.5468/ogs.2014.57.4.266.
Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Pusan National University Hospital, Pusan National University School of Medicine, and Biomedical Research Institute and Pusan Cancer Center, Busan, Korea. ghkim@pusan.ac.kr
- 2Department of Medicine, Pusan National University Graduate School of Medicine, Busan, Korea.
- 3Department of Pathology, Pusan National University School of Medicine, Busan, Korea.
- KMID: 1841537
- DOI: http://doi.org/10.5468/ogs.2014.57.4.266
Abstract
OBJECTIVE
The aim of this study was to determine the distribution of T-lymphocytes and their relationship with clinicopathologic factors in endometrial carcinoma.
METHODS
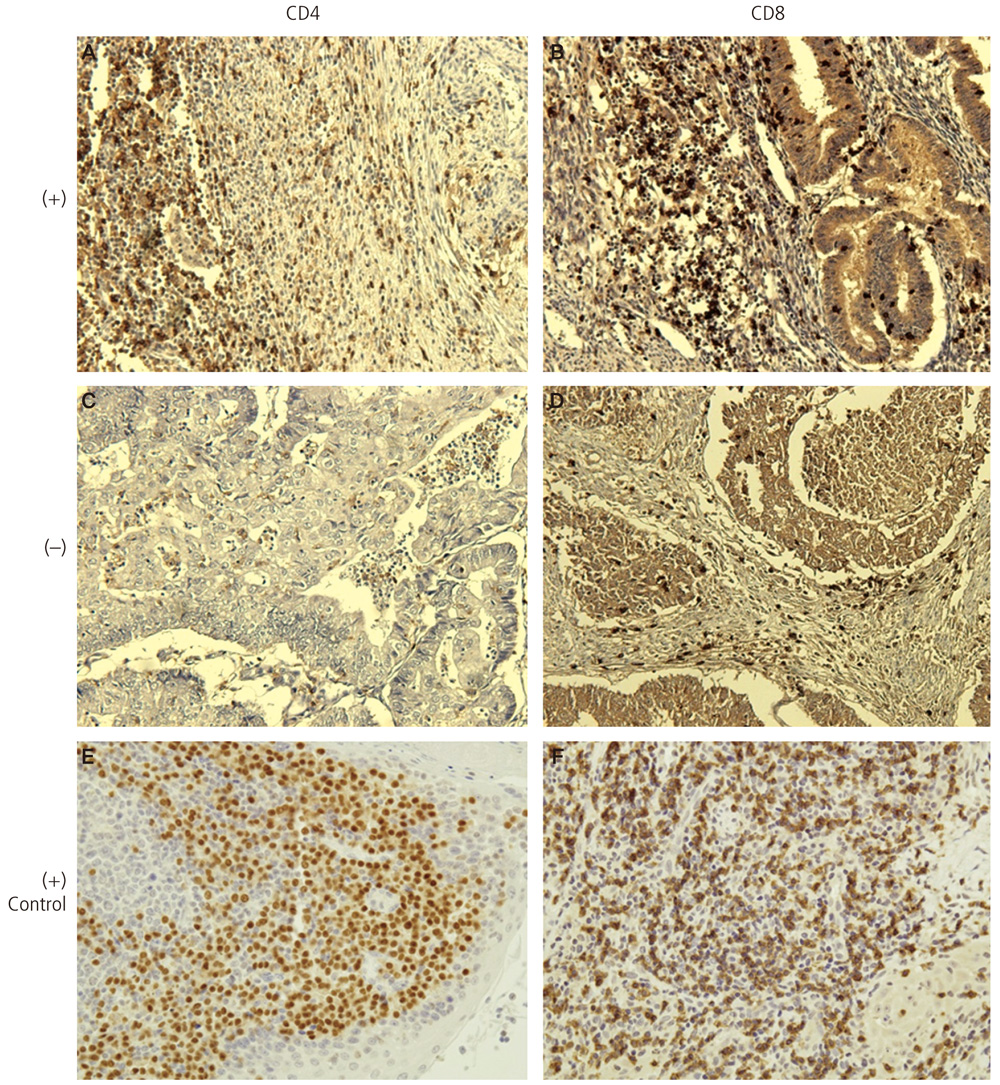
Samples were collected from 89 patients with endometrial endometrioid adenocarcinoma treated in Pusan National University Hospital from 2004 to 2011. Normal endometrial tissues were obtained from 30 hysterectomized women with benign adnexal masses and served as controls. Paraffin-embedded sections were immunohistochemically stained for CD8 (cytotoxic) and CD4 (helper) T-lymphocytes. The relationship of these cells with stage, histological grade, myometrial invasion, and lymph node metastasis was analyzed.
RESULTS
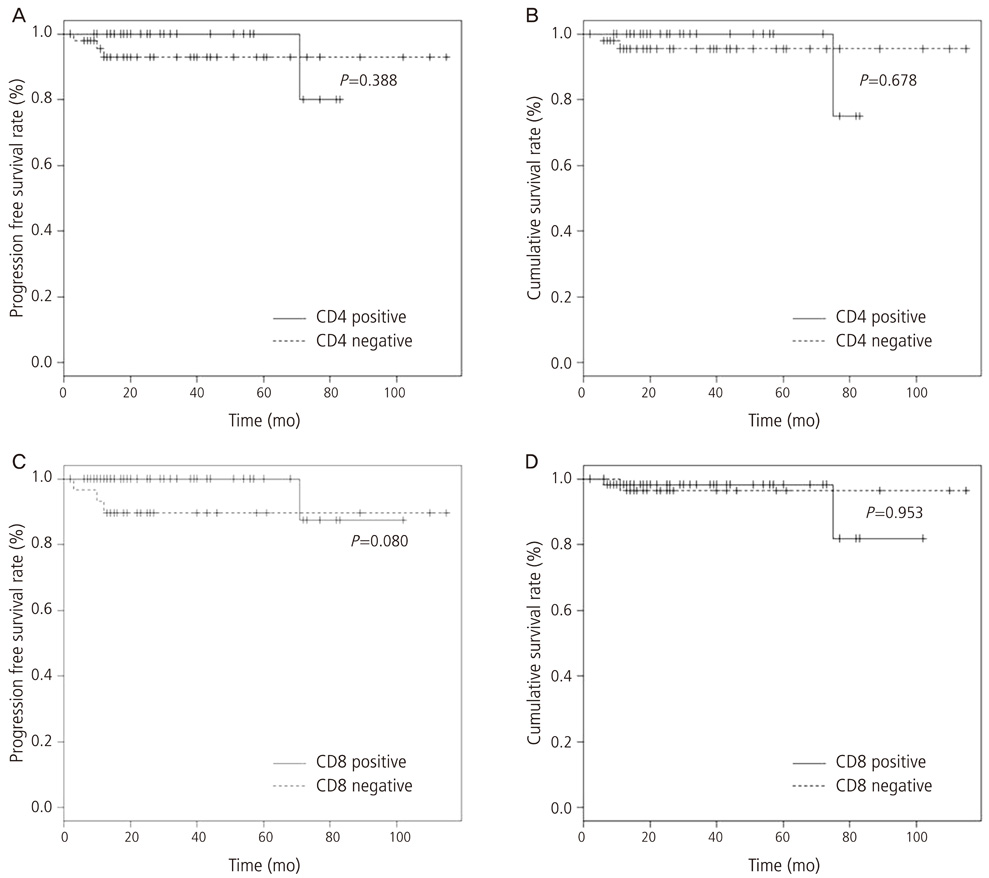
The proportion of CD8+ and CD4+ lymphocytes in the endometrial endometrioid adenocarcinoma tissues was 67.4% (60/89) and 44.9% (40/89), respectively, which was significantly higher (P<0.05) than in the control group. The extent of CD8+ lymphocyte expression was negatively correlated with histologic grade, myometrial invasion, and lymph node metastasis. The proportion of infiltration of the CD4+ lymphocytes was negatively correlated with histologic grade and myometrial invasion.
CONCLUSION
The high rate of infiltration of T-lymphocytes was negatively correlated with histologic grade, myometrial invasion, and lymph node metastasis. Our findings suggest that tumor-infiltrating T-lymphocytes may be used as pathologic prognostic factors in endometrial carcinoma.
MeSH Terms
Figure
Reference
-
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.2. Van der Horst PH, Wang Y, Vandenput I, Kuhne LC, Ewing PC, van Ijcken WF, et al. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One. 2012; 7:e30840.3. Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004; 10:4450–4456.4. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003; 348:203–213.5. Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998; 58:3491–3494.6. Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008; 14:2310–2317.7. Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007; 13:902–911.8. Bontkes HJ, de Gruijl TD, Walboomers JM, van den Muysenberg AJ, Gunther AW, Scheper RJ, et al. Assessment of cytotoxic T-lymphocyte phenotype using the specific markers granzyme B and TIA-1 in cervical neoplastic lesions. Br J Cancer. 1997; 76:1353–1360.9. Yakirevich E, Izhak OB, Rennert G, Kovacs ZG, Resnick MB. Cytotoxic phenotype of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Mod Pathol. 1999; 12:1050–1056.10. Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001; 61:3932–3936.11. Clark WH Jr, Elder DE, Guerry D 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989; 81:1893–1904.12. Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007; 67:9518–9527.13. Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004; 24(5C):3335–3342.14. Wallace AE, Sales KJ, Catalano RD, Anderson RA, Williams AR, Wilson MR, et al. Prostaglandin F2alpha-F-prostanoid receptor signaling promotes neutrophil chemotaxis via chemokine (C-X-C motif) ligand 1 in endometrial adenocarcinoma. Cancer Res. 2009; 69:5726–5733.15. Giatromanolaki A, Bates GJ, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, et al. The presence of tumor-infiltrating FOXP3+ lymphocytes correlates with intratumoral angiogenesis in endometrial cancer. Gynecol Oncol. 2008; 110:216–221.16. Wallace AE, Gibson DA, Saunders PT, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010; 206:141–157.17. Ferguson A, Moore M, Fox H. Expression of MHC products and leucocyte differentiation antigens in gynaecological neoplasms: an immunohistological analysis of the tumour cells and infiltrating leucocytes. Br J Cancer. 1985; 52:551–563.18. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006; 16:115–123.19. Ohno Y, Ohno S, Suzuki N, Kamei T, Inagawa H, Soma G, et al. Role of cyclooxygenase-2 in immunomodulation and prognosis of endometrial carcinoma. Int J Cancer. 2005; 114:696–701.20. Ribatti D, Nico B, Finato N, Crivellato E. Tryptase-positive mast cells and CD8-positive T cells in human endometrial cancer. Pathol Int. 2011; 61:442–444.21. Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003; 171:5051–5063.22. De Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009; 114:105–110.23. Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993; 11:403–450.24. Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE. Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol. 1995; 100:164–172.25. Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008; 14:3372–3379.26. Arafa M, Boniver J, Delvenne P. Progression model tissue microarray (TMA) for the study of uterine carcinomas. Dis Markers. 2010; 28:267–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- S100 expression in dendritic cells is inversely correlated with tumor grade in endometrial carcinoma
- A Case of Concurrent Uterine Malignant Mixed M llerian Tumor and Endometrial Adenocarcinoma
- Analysis of Microsatellite Instability and hMLH1, hMSH2 and Bax Expression in Sporadic Endometrioid Endometrial Adenocarcinoma
- Overexpression of Hepatocyte Growth Factor and c-Met as well as Angiogenesis in Endometrial Hyperplasia and Adenocarcinoma
- Endometrial Mucinous Adenocarcinoma with Extensive Squamous Differentiation: A Case Report