Korean J Physiol Pharmacol.
2008 Aug;12(4):149-153. 10.4196/kjpp.2008.12.4.149.
Expression of Endothelin-1 and Its Receptors in Cisplatin-Induced Acute Renal Failure in Mice
- Affiliations
-
- 1Department of Physiology, Kosin University College of Medicine, Busan, Korea. dwahn@kosin.ac.kr
- KMID: 1838352
- DOI: http://doi.org/10.4196/kjpp.2008.12.4.149
Abstract
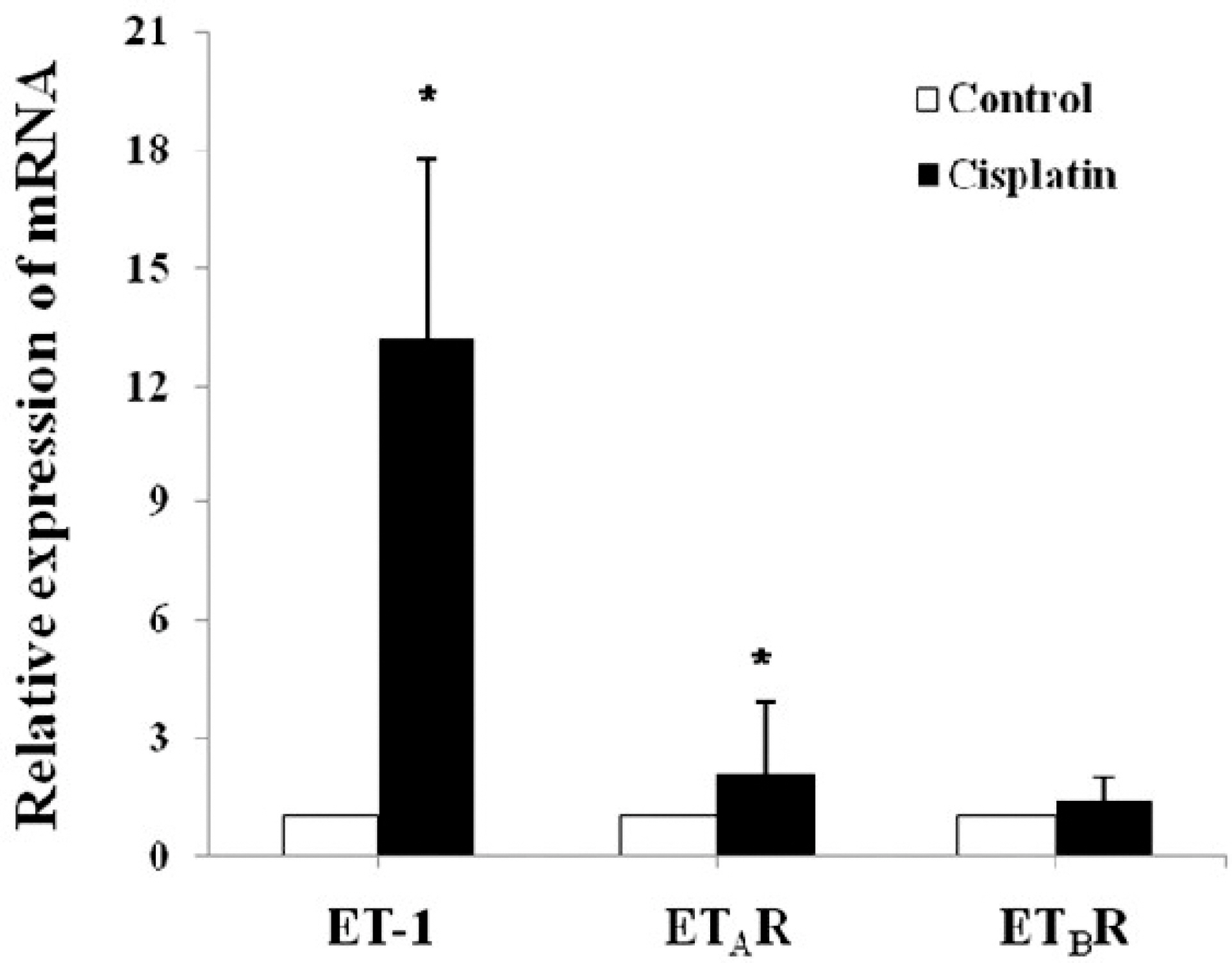
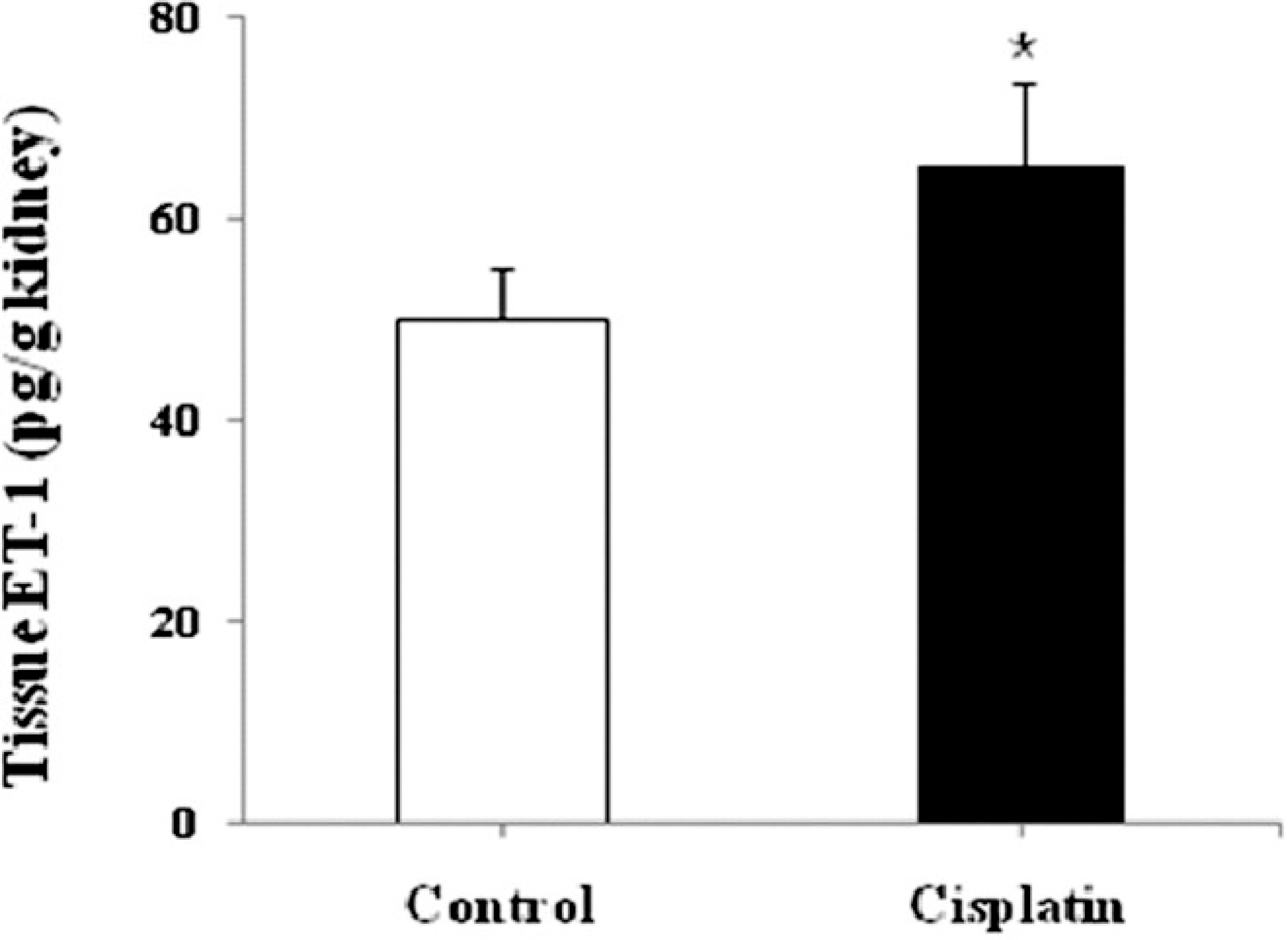
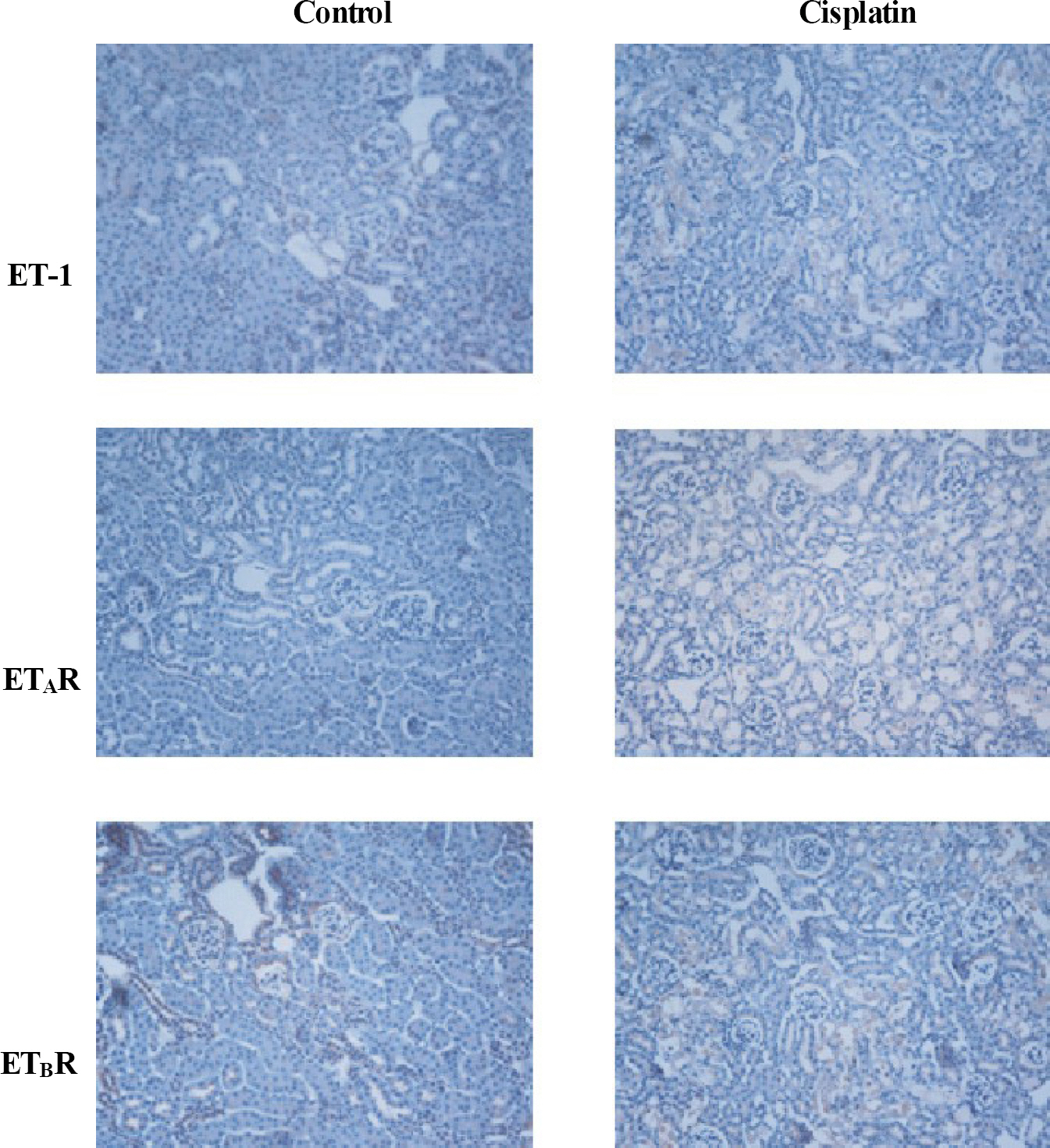
- Endothelin-1 (ET-1) is unequivocally elevated in the kidney with ischemic acute renal failure (ARF), whereas ET receptors (ET(A)R and ET(B)R) are variably expressed. Although renal functional and structural changes are similar between ischemic and nephrotoxic ARF, there are few reports on the alteration in the ET system in nephrotoxic ARF. This study was, therefore, undertaken to investigate changes in renal expression of ET-1 and its receptors in nephrotoxic ARF induced by cisplatin. Mice were intraperitoneally injected with 16 mg of cisplatin/kg at a single dose, and the expression of mRNA and protein was then quantified by real-time RT-PCR and Western blot, respectively. Immunohistochemistry was conducted for localization. Three days after treatment, ET-1 transcript in cisplatin- treated mice was thirteen times higher than that in controls, whereas ET-1 peptide was increased by 1.5-fold. Cisplatin caused a 2-fold increase in the levels of ET(A)R mRNA and protein. Most of the increased immunoreactive ET-1 and ET(A)R were localized in damaged tubules. Neither the expression of ET(B)R mRNA nor the abundance and immunoreactive level of ET(B)R protein were changed. The findings suggest that the individual components of the renal ET system are differentially regulated in cisplatin-induced nephrotoxic ARF.
MeSH Terms
Figure
Reference
-
Ahn D., Ge Y., Stricklett PK., Gill P., Taylor D., Hughes AK., Yanagisawa M., Miller L., Nelson RD., Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 114:504–511. 2004.
ArticleBirck R., Knoll T., Braun C., Kirchengast M., Munter K., van der Woude FJ., Rohmeiss P. Improvement of postischemic acute renal failure with the novel orally active endothelin-A receptor antagonist LU 135252 in the rat. J Cardiovasc Pharmacol. 32:80–86. 1998.
ArticleBraun C., Conzelmann T., Vetter S., Schaub M., Back WE., Yard B., Kirchengast M., Tullius SG., Schnulle P., van der Woude FJ., Rohmeiss P. Prevention of chronic renal allograft rejection in rats with an oral endothelin A receptor antagonist. Transplantation. 68:739–746. 1999.Chatziantoniou C., Dussaule JC. Insights into the mechanisms of renal fibrosis: is it possible to achieve regression? Am J Physiol Renal Physiol. 289:F227–234. 2005.
ArticleDeng DX., Jiang J., Garcia B., Zhong R., Chakrabarti S. Endothelin-1, endothelin-3 and their receptors (endothelin (A) and endothelin (B)) in chronic renal transplant rejection in rats. Transpl Int. 13:175–182. 2000.Forbes JM., Jandeleit-Dahm K., Allen TJ., Hewitson TD., Becker GJ., Jones CL. Endothelin and endothelin A/B receptors are increased after ischaemic acute renal failure. Exp Nephrol. 9:309–316. 2001.
ArticleFrancis BN., Abassi Z., Heyman S., Winaver J., Hoffman A. Differential regulation of ETA and ETB in the renal tissue of rats with compensated and decompensated heart failure. J Cardiovasc Pharmacol. 44:S362–S365. 2004.
ArticleGellai M., Jugus M., Fletcher T., DeWolf R., Nambi P. Reversal of postischemic acute renal failure with a selective endothelinA receptor antagonist in the rat. J Clin Invest. 93:900–906. 1994.
ArticleHoffman A., Grossman E., Goldstein DS., Gill JR Jr. Keiser HR. Urinary excretion rate of endothelin-1 in patients with essential hypertension and salt sensitivity. Kidney Int. 45:556–560. 1994.Kuro T., Kohnou K., Kobayashi Y., Takaoka M., Opgenorth TJ., Wessale JL., Matsumura Y. Selective antagonism of the ETA receptor, but not the ETB receptor, is protective against ischemic acute renal failure in rats. Jpn J Pharmacol. 82:307–316. 2000.
ArticleNakamura T., Ebihara I., Fukui M., Osada S., Tomino Y., Masaki T., Goto K., Furuichi Y., Koide H. Modulation of glomerular endothelin and endothelin receptor gene expression in aminonucleoside- induced nephrosis. J Am Soc Nephrol. 5:1585–1590. 1995.Nelson J., Bagnato A., Battistini B., Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 3:110–116. 2003.
ArticleOng AC., Newby LJ., Dashwood MR. Expression and cellular localisation of renal endothelin-1 and endothelin receptor subtypes in autosomal-dominant polycystic kidney disease. Nephron Exp Nephrol. 93:e80. 2003.
ArticleRamesh G., Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 110:835–842. 2002.Roubert P., Gillard-Roubert V., Pourmarin L., Cornet S., Guilmard C., Plas P., Pirotzky E., Chabrier PE., Braquet P. Endothelin receptor subtypes A and B are up-regulated in an experimental model of acute renal failure. Mol Pharmacol. 45:182–188. 1994.Schellmann RG., Kelly KJ. Pathophysiology of nephrotoxic acute renal failure. Schrier RW, editor. ed,. Atlas of Diseases of the Kidney. 1st ed.Current medicine Inc.;Philadelphia: p. p. 15.1–15.14. 1999.Sheridan AM., Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 7−21:2001.
ArticleShimizu T., Kuroda T., Ikeda M., Hata S., Fujimoto M. Potential contribution of endothelin to renal abnormalities in glycerol-induced acute renal failure in rats. J Pharmacol Exp Ther. 286:977–983. 1998.Stein JH., Lifschitz MD., Barnes LD. Current concepts on the pathophysiology of acute renal failure. Am J Physiol. 234:F171–181. 1978.
ArticleTakaoka M., Kuro T., Matsumura Y. Role of endothelin in the pathogenesis of acute renal failure. Drug News Perspect. 13:141–146. 2000.
ArticleYoshimura A., Iwasaki S., Inui K., Ideura T., Koshikawa S., Yanagisawa M., Masaki T. Endothelin-1 and endothelin B type receptor are induced in mesangial proliferative nephritis in the rat. Kidney Int. 48:1290–1297. 1995.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Investigation of the Expression of Fractalkine and the Infiltration Characteristics of Fractalkine Receptor Positive Cells and Macrophages in Cisplatin-induced Acute Renal Failure in Mice
- Role of IL-1alpha in Cisplatin-Induced Acute Renal Failure in Mice
- Protective effects of a mineral aqueous solution on toxicity in mouse liver and kidney
- Changes in Endothelin Receptor Type B and Neuronal Nitric Oxide Synthase in Puromycin Aminonucleoside-Induced Nephrotic Syndrome
- Post-treatment Effects of Erythropoietin and Nordihydroguaiaretic Acid on Recovery from Cisplatin-induced Acute Renal Failure in the Rat