Korean J Obstet Gynecol.
2012 Jan;55(1):8-16. 10.5468/KJOG.2012.55.1.8.
Immunohisochemical study of BRCA1, BRCA2, and poly (ADP-ribose) polymerase-1 in ovarian tumors
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Sungae Hospital, Seoul, Korea.
- 2Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea. taejlee@cau.ac.kr
- KMID: 1836757
- DOI: http://doi.org/10.5468/KJOG.2012.55.1.8
Abstract
OBJECTIVE
BRCA1 and BRCA2 are putative tumor suppressor gene responsible for hereditary ovarian cancer syndrome. Both BRCA1 and BRCA2 are involved in maintaining genome integrity in DNA repair. In the complex DNA repair related proteins such as poly (ADP-ribose) polymerase-1 (PARP1) have been less studied in ovarian cancer. The aim of our work is to analysis the protein expression of BRCA1, BRCA2 and PARP1 in ovarian tumors including benign, borderline, malignant tumors.
METHODS
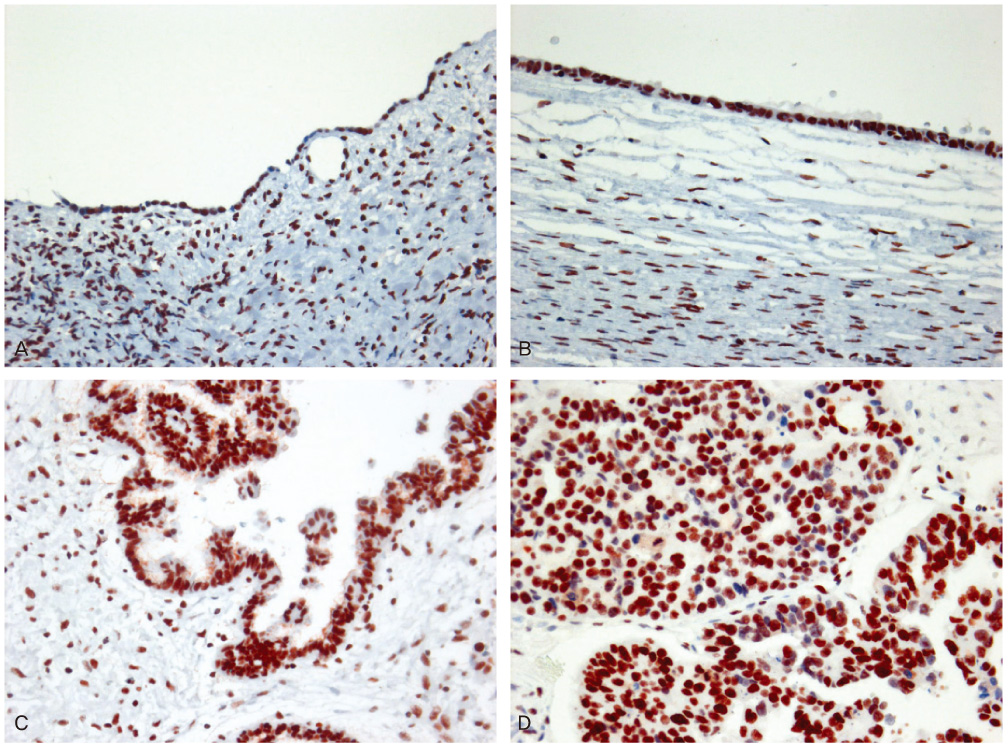
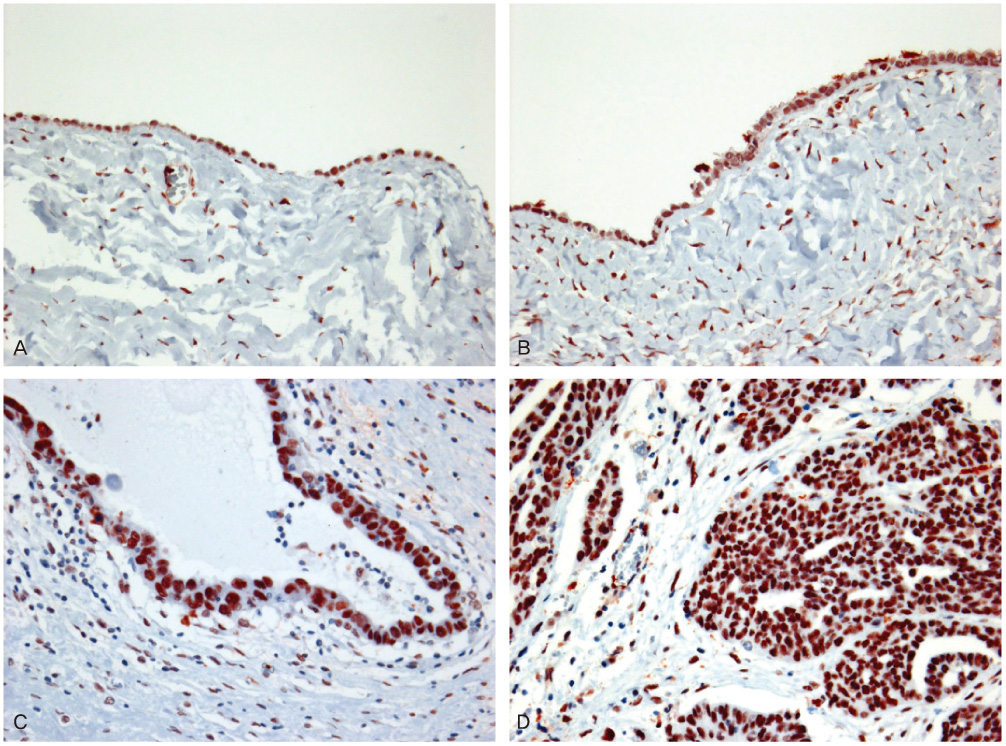
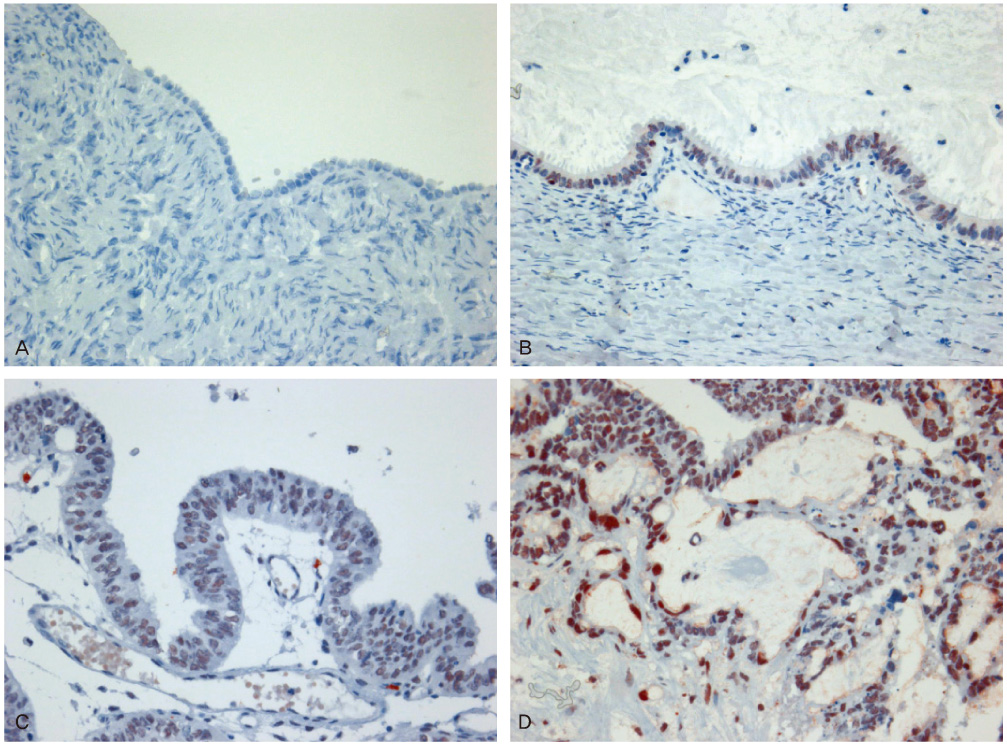
We selected 10 cases of normal tissue, 24 cases of borderline tumors, 40 cases of benign tumors, and 52 cases of carcinomas in ovary. Immunohistochemistry for BRCA1, BRCA2, and PARP1 was performed on paraffin sections.
RESULTS
Negative expression of BRCA1 and BRCA2 was found in 12.5% and 22.5% of benign tumors, 25.0% and 33.3% of borderline tumors, and 36.5% and 40.4% of carcinomas, respectively. Expression of PARP1 was detected in 22.5% of benign tumors, 58.3% of borderline tumors, and 69.2% of carcinomas. Negative expression of BRCA1 with BRCA2 correlated with the expression of PARP1.
CONCLUSION
Negative expression of BRCA1 with BRCA2 and PARP1 expression may play an important role in the development of ovarian tumor.
Keyword
MeSH Terms
Figure
Reference
-
1. Park CS, Kim JK. Expression of BRCA1 transcripts and protein in sporadic ovarian cancer. Korean J Obstet Gynecol. 1998. 41:2131–2145.2. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004. 4:665–676.3. Zikan M, Janatova M, Pavlista D, Pohlreich P. High frequency of BRCA1/2 and p53 somatic inactivation in sporadic ovarian cancer. J Genet. 2007. 86:169–171.4. Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, et al. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004. 202:215–223.5. Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999. 22:37–43.6. Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999. 4:511–518.7. Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001. 7:263–272.8. Patel KJ, Joenje H. Fanconi anemia and DNA replication repair. DNA Repair (Amst). 2007. 6:885–890.9. Venkitaraman AR. Modifying chromatin architecture during the response to DNA breakage. Crit Rev Biochem Mol Biol. 2010. 45:2–13.10. Shaheen M, Allen C, Nickoloff JA, Hromas R. Synthetic lethality: exploiting the addiction of cancer to DNA repair. Blood. 2011. 117:6074–6082.11. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005. 434:913–917.12. Sugimura K, Takebayashi S, Taguchi H, Takeda S, Okumura K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J Cell Biol. 2008. 183:1203–1212.13. Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, Helleday T. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol Cell Biol. 2005. 25:7158–7169.14. Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park). 2008. 22:1233–1239.15. Alberg AJ, Helzlsouer KJ. Epidemiology, prevention, and early detection of breast cancer. Curr Opin Oncol. 1997. 9:505–511.16. Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998. 2:317–328.17. Koul A, Malander S, Loman N, Pejovic T, Heim S, Willen R, et al. BRCA1 and BRCA2 mutations in ovarian cancer: Covariation with specific cytogenetic features. Int J Gynecol Cancer. 2000. 10:289–295.18. Chan KY, Ozcelik H, Cheung AN, Ngan HY, Khoo US. Epigenetic factors controlling the BRCA1 and BRCA2 genes in sporadic ovarian cancer. Cancer Res. 2002. 62:4151–4156.19. Hedenfalk IA. Gene expression profiling of hereditary and sporadic ovarian cancers reveals unique BRCA1 and BRCA2 signatures. J Natl Cancer Inst. 2002. 94:960–961.20. Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003. 97:2187–2195.21. Gras E, Cortes J, Diez O, Alonso C, Matias-Guiu X, Baiget M, et al. Loss of heterozygosity on chromosome 13q12-q14, BRCA-2 mutations and lack of BRCA-2 promoter hypermethylation in sporadic epithelial ovarian tumors. Cancer. 2001. 92:787–795.22. D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl) ation reactions in the regulation of nuclear functions. Biochem J. 1999. 342:249–268.23. Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis: caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999. 274:22932–22940.24. Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005. 4:421–440.25. Hassa PO, Covic M, Bedford MT, Hottiger MO. Protein arginine methyltransferase 1 coactivates NF-kappaB-dependent gene expression synergistically with CARM1 and PARP1. J Mol Biol. 2008. 377:668–678.26. Timinszky G, Till S, Hassa PO, Hothorn M, Kustatscher G, Nijmeijer B, et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat Struct Mol Biol. 2009. 16:923–929.27. Zahradka P, Ebisuzaki K. A shuttle mechanism for DNA-protein interactions: the regulation of poly(ADP-ribose) polymerase. Eur J Biochem. 1982. 127:579–585.28. Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009. 361:123–134.29. Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010. 28:2512–2519.30. Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer. 2004. 4:814–819.31. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010. 10:293–301.32. Pizem J, Popovic M, Cör A. Expression of Gli1 and PARP1 in medulloblastoma: an immunohistochemical study of 65 cases. J Neurooncol. 2011. 103:459–467.33. Piao L, Nakagawa H, Ueda K, Chung S, Kashiwaya K, Eguchi H, et al. C12orf48, termed PARP-1 binding protein, enhances poly(ADP-ribose) polymerase-1 (PARP-1) activity and protects pancreatic cancer cells from DNA damage. Genes Chromosomes Cancer. 2011. 50:13–24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- BRCA1/BRCA2 Pathogenic Variant Breast Cancer: Treatment and Prevention Strategies
- Expression of DNA Damage Response Proteins and Associations with Clinicopathologic Characteristics in Chinese Familial Breast Cancer Patients with BRCA1/2 Mutations
- One Case of BRCA2 Germline Mutation Ovarian Cancer Mother and Carrier Daughter found by Genetic Counseling
- The Important Role of Poly ADP-Ribose Polymerase Inhibitor in Prostate Cancer
- BRCA1 Reversion Mutation Confers Resistance to Olaparib and Camrelizumab in a Patient with Breast Cancer Liver Metastasis