Korean J Hematol.
2012 Dec;47(4):267-272. 10.5045/kjh.2012.47.4.267.
Prognostic significance of gelsolin and MMP12 in Langerhans cell histiocytosis
- Affiliations
-
- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Ulsan College of Medicine & Asan Medical Center, Seoul, Korea.
- 2Department of Pediatrics, Hanyang University Medical Center, Seoul, Korea.
- 3Department of Surgery, Pittsburgh University, Pittsburgh, PA, USA. nel1205@hanmail.net
- 4Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Chungnam National University College of Medicine, Daejeon, Korea.
- KMID: 1832105
- DOI: http://doi.org/10.5045/kjh.2012.47.4.267
Abstract
- BACKGROUND
Gelsolin and matrix metalloproteinase 12 (MMP12) expression has been reported in Langerhans cell histiocytosis (LCH), but the clinical significance of this expression is unknown. We investigated the associations of these proteins with clinical manifestations in patients diagnosed with LCH.
METHODS
We performed a retrospective analysis of clinical data from patients diagnosed with LCH and followed up between 1998 and 2008. Available formalin-fixed, paraffin-embedded specimens were used for gelsolin and MMP12 immunohistochemical staining. We analyzed the expression levels of these proteins and their associations with LCH clinical features.
RESULTS
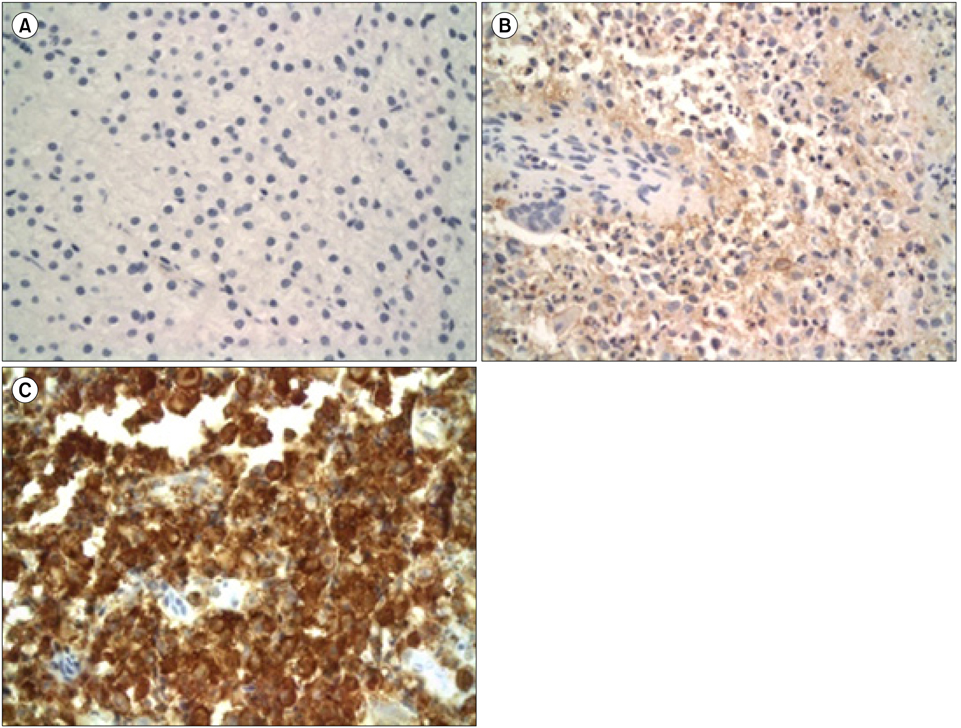
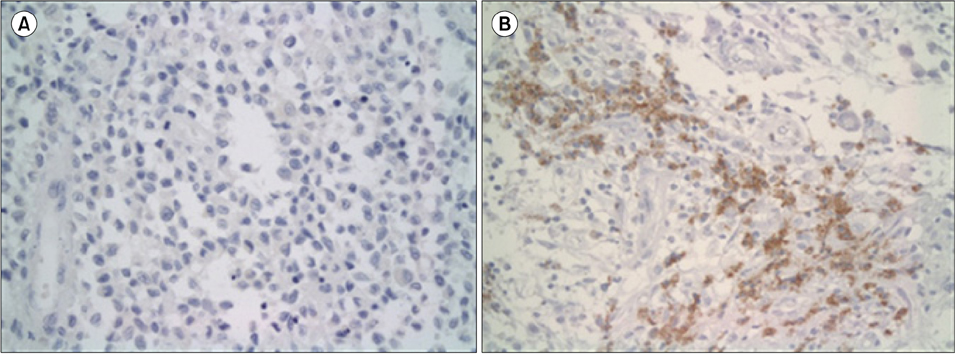
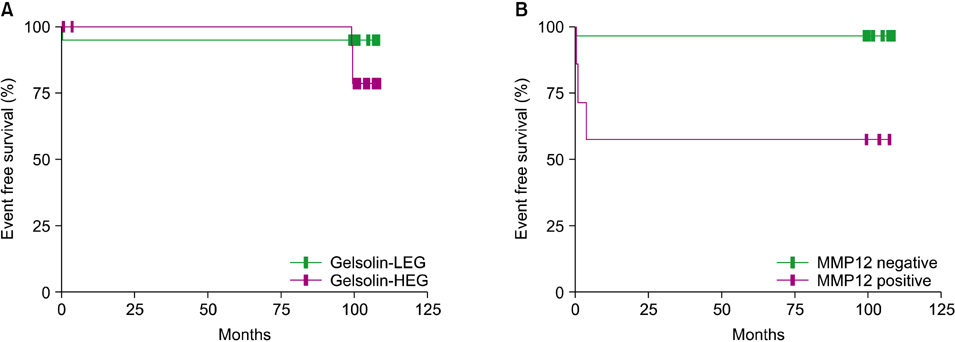
Specimens from 36 patients (20 males, 16 females) with a diagnosis of LCH based on CD1a positivity with clinical manifestations were available for immunohistochemical staining. Median patient age was 62 months (range, 5 to 207). The expression of gelsolin varied; it was high in 17 patients (47.2%), low in 11 patients (30.6%), and absent in 8 patients (22.2%). The high gelsolin expression group had a higher tendency for multi-organ and risk organ involvement, although the trend was not statistically significant. MMP12 was detected only in 7 patients (19.4%) who showed multi-system involvement (P=0.018) and lower event-free survival (P=0.002) in comparison to patients with negative MMP12 staining.
CONCLUSION
Gelsolin and MMP12 expression may be associated with the clinical course of LCH, and MMP12 expression may be particularly associated with severe LCH. Further studies of larger populations are needed to define the precise role and significance of gelsolin and MMP12 in the pathogenesis of LCH.
Keyword
MeSH Terms
Figure
Reference
-
1. Rust R, Kluiver J, Visser L, et al. Gene expression analysis of dendritic/Langerhans cells and Langerhans cell histiocytosis. J Pathol. 2006. 209:474–483.
Article2. Sholl LM, Hornick JL, Pinkus JL, Pinkus GS, Padera RF. Immunohistochemical analysis of langerin in langerhans cell histiocytosis and pulmonary inflammatory and infectious diseases. Am J Surg Pathol. 2007. 31:947–952.
Article3. Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008. 50:304–307.
Article4. Petersen BL, Rengtved P, Bank MI, Carstensen H. High expression of markers of apoptosis in Langerhans cell histiocytosis. Histopathology. 2003. 42:186–193.
Article5. Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol. 1999. 11:103–108.
Article6. Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999. 274:33179–33182.
Article7. Witke W, Li W, Kwiatkowski DJ, Southwick FS. Comparisons of CapG and gelsolin-null macrophages: demonstration of a unique role for CapG in receptor-mediated ruffling, phagocytosis, and vesicle rocketing. J Cell Biol. 2001. 154:775–784.8. Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005. 100:Suppl 1. 167–172.
Article9. Gueders MM, Foidart JM, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006. 533:133–144.
Article10. Kerkelä E, Ala-aho R, Klemi P, et al. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J Pathol. 2002. 198:258–269.
Article11. Kerkelä E, Ala-Aho R, Jeskanen L, et al. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000. 114:1113–1119.
Article12. Balaz P, Friess H, Kondo Y, Zhu Z, Zimmermann A, Büchler MW. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann Surg. 2002. 235:519–527.
Article13. Ladisch S, Gadner H, Aricò M, et al. The Histiocyte Society. LCH-I: a randomized trial of etoposide vs. vinblastine in disseminated Langerhans cell histiocytosis. Med Pediatr Oncol. 1994. 23:107–110.
Article14. Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997. 29:157–166.15. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008. 25:291–295.
Article16. Garabedian L, Struyf S, Opdenakker G, Sozzani S, Van Damme J, Laureys G. Langerhans cell histiocytosis: a cytokine/chemokine-mediated disorder? Eur Cytokine Netw. 2011. 22:148–153.
Article17. Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010. 184:4557–4567.
Article18. Bank MI, Rengtved P, Carstensen H, Petersen BL. p53 expression in biopsies from children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002. 24:733–736.
Article19. Schouten B, Egeler RM, Leenen PJ, Taminiau AH, van den Broek LJ, Hogendoorn PC. Expression of cell cycle-related gene products in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002. 24:727–732.
Article20. Kim SY, Kim HJ, Kim HJ, et al. Role of p16 in the pathogenesis of Langerhans cell histiocytosis. Korean J Hematol. 2010. 45:247–252.
Article21. Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004. 61:2614–2623.
Article22. Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008. 50:304–307.
Article23. Geng YJ, Azuma T, Tang JX, et al. Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur J Cell Biol. 1998. 77:294–302.
Article24. Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997. 278:294–298.
Article25. Zyada MM. Expression of matrix metalloproteinase-9 and significance of a macrophage assay in eosinophilic granuloma. Ann Diagn Pathol. 2009. 13:367–372.
Article26. Hayashi T, Rush WL, Travis WD, Liotta LA, Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary Langerhans' cell granulomatosis. Arch Pathol Lab Med. 1997. 121:930–937.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Pneumothorax due to Pulmonary Invasion in Multisystemic Langerhans Cell Histiocytosis: A case report
- Pulmonary Langerhans Cell Histiocytosis Accompanied by Active Pulmonary Tuberculosis
- A Case of Orbital Langerhans' cell histiocytosis
- A Case of Pulmonary Langerhans Cell Histiocytosis with Pneumothorax
- Adult Scapular Langerhans Cell Histiocytosis Mistaken for Acute Osteomyelitis