J Lung Cancer.
2007 Dec;6(2):85-90. 10.6058/jlc.2007.6.2.85.
Efficacy and Safety of Weekly Low Dose Paclitaxel and Cisplatin as First Line Therapy in Advanced NSCLC of Elderly Patients
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Konyang University, Daejeon, Korea. sk1609@hanmail.net
- 2Department of Chest Surgery, College of Medicine, Konyang University, Daejeon, Korea.
- 3Department of Therapeutic Radiology and Oncology, College of Medicine, Konyang University, Daejeon, Korea.
- 4Department of Radiology, College of Medicine, Konyang University, Daejeon, Korea.
- KMID: 1820250
- DOI: http://doi.org/10.6058/jlc.2007.6.2.85
Abstract
-
PURPOSE : Anti-cancer chemotherapeutic agents act by inhibiting tumor cell proliferation through cytotoxic action, therefore the generally tolerated maximum dose is administered to patients. However, this often results in the production of undesirable toxicities, such as bone marrow suppression, and a long interruption of treatment is necessary for recovery to occur before additional cycles of treatment are administered. Paclitaxel and cisplatin are well known effective chemotherapeutic agents used for the treatment of Non-Small Cell Lung Cancer (NCSLC), however, they have substantial toxicities. To evaluate efficacy and safety of a therapy consisting of a weekly low dose of paclitaxel and therapy in elderly patients with advanced NSCLC.
MATERIALS AND METHODS
: Thirteen treatment-naive, elderly patients over 65 years old who were diagnosed with stage IV NSCLC at Konyang University from April 2005 to October 2006 were enrolled in the present study. Paclitaxel at a dose of 55 mg/m2 in combination with cisplatin at a dose of 20 mg/m2 was administered intravenously on day 1, 8, and 15 with 1 week of interruption for a total of six cycles of chemotherapy.
RESULTS
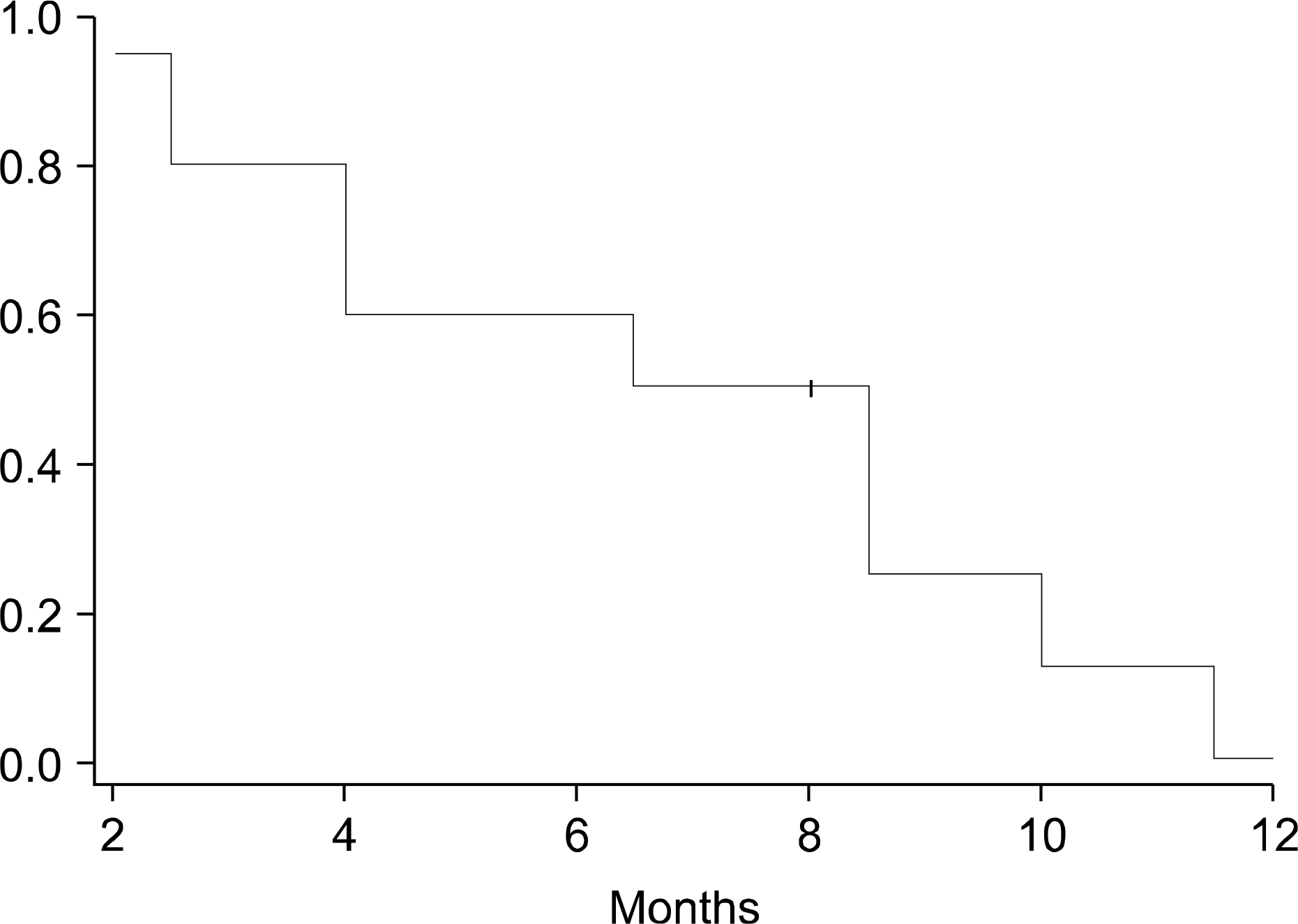
: The mean age of the ten patients included in this study was 69.5 years. Following treatment, 50 % of the patients exhibited a partial response to treatment, whereas the disease remained stable in 40% of the patients, and progressed in 10% of the patients. The median survival time (Kaplan-Meier method) was 15 months (4~24 months), and the 6-month, 1-year, and 2-year survival rates were 80%, 50%, and 10%, respectively. The median progression survival time was 8 months (2~14 months) and the 6- and 12-month progression free survival rates were 60% and 10%, respectively. Grade 3 neutropenia occurred in only 1 case (10%).
CONCLUSION
: The results of this study indicated that chemotherapy consisting of a weekly low dose of paclitaxel and cisplatin could be more effective and have lesser toxicity when administered to elderly patients with advanced NSCLC. In addition, this treatment regimen showed a promising response rate
Keyword
MeSH Terms
Figure
Reference
-
References
1. Report of the cause in Korea. Ministry of Health and Welfare;2004.2. annual report of Korea Central Cancer Registry (published in 2004). Available form URL:. http://www.ncc.re.kr.3. Jo HJ, Kim HK, Song SH, et al. Two years' result of lung cancer registry in St. Vincent's Hospital. Cancer Reser Treat. 2002; 34(Suppl 1):143. (#190).4. Woo CM, Kim SY, Lee SA, et al. Paclitaxel for elderly patients with advanced NSCLC. The Korean Journal of Medicine. 2006; 70:183–189.5. Socinski MA. Single-agent paclitaxel in the treatment of advanced nonsmall cell lung cancer. Oncologist. 1999; 4:408–416.
Article6. Cardenal F, Lopez-Cabrerizo MP, Antó n A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic nonsmall-cell lung cancer. J Clin Oncol. 1999; 17:12–18.
Article7. Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced nonsmall-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000; 18:623–631.
Article8. Giaccone G, Splinter TA, Debruyne C, et al. Randomized study of paclitaxel-cisplatin versus cisplatin-teniposide in patients with advanced nonsmall-cell lung cancer. J Clin Oncol. 1998; 16:2133–2141.9. Arbuck SG. Paclitaxel: what schedule? What dose? J Clini Oncol. 1994; 12:233–236.
Article10. Rowinsky EK, Jiroutek M, Bonomi P, Johnson D, Baker SD. Paclitaxel steady-state plasma concentration as a determinant of disease outcome and toxicity in lung cancer patients treated with paclitaxel and cisplatin. Clin Cancer Res. 1999; 5:767–774.11. Klastersky J, Sculier JP, Ravez P, et al. A randomized study comparing a high and a standard dose of cispaltin in combination with etoposide in the treatment of advanced non-small-cell lung carcinoma. J Clin Oncol. 1986; 4:1780–1786.12. Kim SW, Suh C, Lee SD, et al. Weekly low dose palclitaxel and cispaltin as first-line chemotherapy for advanced non-small cell lung cancer. Lung Cancer. 2003; 41:221–226.13. The Elderly Lung Cancer Vinorelbine Italian Study Group. Effects of vinorelbine of quality of life and survival of elderly patients with advenced nonsmall-cell lung cancer. J Natl Cancer Inst. 1999; 91:66–72.14. Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced nonsmall-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–372.
Article15. Lilenbaum RC, Herndon JE 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced nonsmall-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol. 2005; 23:190–196.
Article16. Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmocokine-tic/phamacodynamic relationships in humans. J Clin Oncol. 1995; 13:180–190.17. Rowinsky E, Mackey M, Sn G. Meta-analysis of paclitaxel doseresponse and dose-intensity in recurrent or refractory ovarian cancer. Proc Am Soc Clin Oncol. 1996; 15:284.18. Yeung TK, Germond C, Chen X, Wang Z. The mode of action of taxol: apoptosis at low concentration and necrosis at high concentration. Biochem Biophys Res Commun. 1999; 263:398–404.
Article19. Gergiadis MS, Schuler BS, Brown JE, et al. Paclitaxel by 96-hour continuous infusion in combination with cisplatin: a phase I trial in patients with advanced lung cancer. J Clin Oncol. 1997; 15:735–743.20. Kim R, Osaki A, Toge T. Pharmacokinetic and biochemical analysis in the treatment of weekly paclitaxel in relapsed breast cancer. Oncol Rel. 2001; 8:1171–1176.
Article21. Aisner J, Belani CP, Kearns C, et al. Feasibility and pharmacokinetics of paclitaxel, carboplatin, and concurrent radiotherapy for regionally advanced squamous cell carcinoma of the head and neck and for regionally advanced non-small-cell lung cancer. Semin Oncol. 1995; 22(5 Suppl. 12):17–21.22. Choy H, Rodriguez RC, Koester S, Hilsenbeck S, Von Hoff DD. Investigation of taxol as a potential radiation sensitizer. Cancer. 1993; 71:3374–3778.
Article23. Kim HK, Kim JS, Ryoo HN, et al. The efficacy and safety of padexol (paclitaxel) and cisplatin for treating advanced nonsmall cell lung cancer. Cancer Res Treat. 2006; 38:66–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of Paclitaxel and Cisplatin as Second-line Chemotherapy in Advanced Non-small Cell Lung Cancer
- Efficacy of Combination Chemotherapy with Paclitaxel and Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer
- Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen?
- Concurrent Chemoradiation with Weekly Paclitaxel in Locally Advanced Non-small Cell Lung Cancer