J Gynecol Oncol.
2014 Jan;25(1):22-29. 10.3802/jgo.2014.25.1.22.
Role of paclitaxel and cisplatin as the neoadjuvant treatment for locally advanced squamous cell carcinoma of the vulva
- Affiliations
-
- 1Gynecologic Oncology Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. raspagliesi@istitutotumori.mi.it
- 2Department of Obstetrics and Gynecology, University of Uruguay School of Medicine, Montevideo, Uruguay.
- KMID: 1811184
- DOI: http://doi.org/10.3802/jgo.2014.25.1.22
Abstract
OBJECTIVE
The therapeutic outcomes of patients with advanced vulvar cancer are poor. Multi-modality treatments including concurrent chemoradiation or different regimens of neoadjuvant chemotherapy (NACT), and surgery have been explored to reduce the extent of surgery and morbidity. The present single-institution trial aimed to evaluate the efficacy and toxicity of paclitaxel and cisplatin in locally advanced vulvar cancer.
METHODS
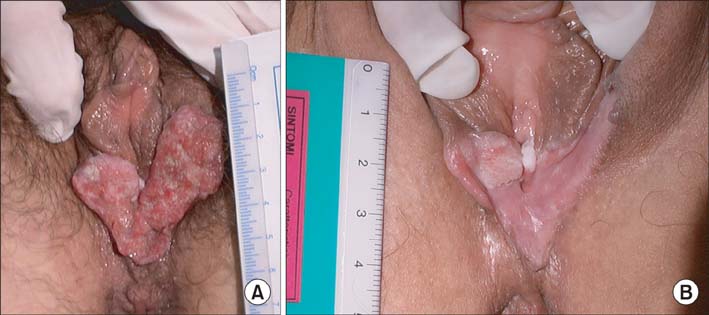
From 2002 to 2009, 10 patients with stage III-IV locally advanced squamous cell carcinoma of the vulva were prospectively treated with 3 courses of paclitaxel-ifosfamide-cisplatin or paclitaxel-cisplatin. Nine of them subsequently underwent radical local excision or radical partial vulvectomy and bilateral inguino-femoral lymphadenectomy.
RESULTS
The clinical response rate of all enrolled patients was 80%, whereas the pathological responses included 1 case with complete remission, 2 with persistent carcinoma in situ, and 6 invasive cancer cases with tumor shrinkage of more than 50%. Four patients had positive nodes. Forty percent of patients experienced grade 3-4 bone marrow toxicity, which was successfully managed with granulocyte-colony stimulating factor, even in cases of elderly patients. Median progression-free survival after surgery was 14 months (range, 5 to 44 months). Six of the 7 recurrent cases were local, and 3 of them were treated with salvage surgery while the other 3 received radiation with or without chemotherapy. After a median follow-up period of 40 months (range, 5 to 112 months), 55.5% of patients remained alive with no evidence of disease, including 2 long-term survivors after recurrence at 5 and 9 years.
CONCLUSION
Based on the high response rate and manageable toxicity, NACT with paclitaxel and cisplatin with or without ifosfamide followed by surgery could be considered as a therapeutic option for locally advanced vulvar cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56:106–130.2. Sturgeon SR, Brinton LA, Devesa SS, Kurman RJ. In situ and invasive vulvar cancer incidence trends (1973 to 1987). Am J Obstet Gynecol. 1992; 166:1482–1485.3. Beller U, Sideri M, Maisonneuve P, Benedet JL, Heintz AP, Ngan HY, et al. Carcinoma of the vulva. J Epidemiol Biostat. 2001; 6:155–173.4. van der Velden J, van Lindert AC, Gimbrere CH, Oosting H, Heintz AP. Epidemiologic data on vulvar cancer: comparison of hospital with population-based data. Gynecol Oncol. 1996; 62:379–383.5. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006; 107:1018–1022.6. Podratz KC, Symmonds RE, Taylor WF. Carcinoma of the vulva: analysis of treatment failures. Am J Obstet Gynecol. 1982; 143:340–351.7. Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, et al. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95:Suppl 1. S7–S27.8. Phillips B, Buchsbaum HJ, Lifshitz S. Pelvic exenteration for vulvovaginal carcinoma. Am J Obstet Gynecol. 1981; 141:1038–1044.9. Cavanagh D, Shepherd JH. The place of pelvic exenteration in the primary management of advanced carcinoma of the vulva. Gynecol Oncol. 1982; 13:318–322.10. Eifel PJ, Berek JS, Thigpen JT. Carcinoma of the vulva. In : De Vita VT, Hellman S, Rosenberg SA, editors. Cancer principles and practice of oncology. New York: Lippincott-Raven;1997. p. 1462–1469.11. de Hullu JA, Oonk MH, van der Zee AG. Modern management of vulvar cancer. Curr Opin Obstet Gynecol. 2004; 16:65–72.12. Shylasree TS, Bryant A, Howells RE. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst Rev. 2011; (4):CD003752.13. Lupi G, Raspagliesi F, Zucali R, Fontanelli R, Paladini D, Kenda R, et al. Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma: a pilot study. Cancer. 1996; 77:1472–1478.14. Landoni F, Maneo A, Zanetta G, Colombo A, Nava S, Placa F, et al. Concurrent preoperative chemotherapy with 5-fluorouracil and mitomycin C and radiotherapy (FUMIR) followed by limited surgery in locally advanced and recurrent vulvar carcinoma. Gynecol Oncol. 1996; 61:321–327.15. Maneo A, Landoni F, Colombo A, Colombo A, Villa A, Caspani G. Randomised study between neoadjuvant chemoradiotherapy and primary surgery for the treatment of advanced vulval cancer [abstract]. Int J Gynecol Cancer. 2003; 13:Suppl 1. 6.16. Zanetta G, Lissoni A, Pellegrino A, Sessa C, Colombo N, Gueli-Alletti D, et al. Neoadjuvant chemotherapy with cisplatin, ifosfamide and paclitaxel for locally advanced squamous-cell cervical cancer. Ann Oncol. 1998; 9:977–980.17. Dimopoulos MA, Papadimitriou CA, Sarris K, Aravantinos G, Kalofonos C, Gika D, et al. Combination of ifosfamide, paclitaxel, and cisplatin for the treatment of metastatic and recurrent carcinoma of the uterine cervix: a phase II study of the Hellenic Cooperative Oncology Group. Gynecol Oncol. 2002; 85:476–482.18. Zanetta G, Fei F, Mangioni C. Chemotherapy with paclitaxel, ifosfamide, and cisplatin for the treatment of squamous cell cervical cancer: the experience of Monza. Semin Oncol. 2000; 27:1 Suppl 1. 23–27.19. Buda A, Fossati R, Colombo N, Fei F, Floriani I, Gueli Alletti D, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol. 2005; 23:4137–4145.20. Lissoni AA, Colombo N, Pellegrino A, Parma G, Zola P, Katsaros D, et al. A phase II, randomized trial of neo-adjuvant chemotherapy comparing a three-drug combination of paclitaxel, ifosfamide, and cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the Snap-02 Italian Collaborative Study. Ann Oncol. 2009; 20:660–665.21. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.22. Raspagliesi F, Hanozet F, Ditto A, Solima E, Zanaboni F, Vecchione F, et al. Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol. 2006; 102:333–337.23. National Cancer Institute, Division of Cancer Treatment and Diagnosis. Common Toxicity Criteria (CTC) ver. 2.0 [Internet]. Bethesda, MD: National Cancer Institute;1999. cited 2013 Nov 20. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.24. van Doorn HC, Ansink A, Verhaar-Langereis M, Stalpers L. Neo-adjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst Rev. 2006; (3):CD003752.25. Moore DH, Ali S, Koh WJ, Michael H, Barnes MN, McCourt CK, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol. 2012; 124:529–533.26. Shimizu Y, Hasumi K, Masubuchi K. Effective chemotherapy consisting of bleomycin, vincristine, mitomycin C, and cisplatin (BOMP) for a patient with inoperable vulvar cancer. Gynecol Oncol. 1990; 36:423–427.27. Benedetti-Panici P, Greggi S, Scambia G, Salerno G, Mancuso S. Cisplatin (P), bleomycin (B), and methotrexate (M) preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol Oncol. 1993; 50:49–53.28. Wagenaar HC, Colombo N, Vergote I, Hoctin-Boes G, Zanetta G, Pecorelli S, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol. 2001; 81:348–354.29. Geisler JP, Manahan KJ, Buller RE. Neoadjuvant chemotherapy in vulvar cancer: avoiding primary exenteration. Gynecol Oncol. 2006; 100:53–57.30. Witteveen PO, van der Velden J, Vergote I, Guerra C, Scarabeli C, Coens C, et al. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: a study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer--Gynaecological Cancer Group). Ann Oncol. 2009; 20:1511–1516.31. Domingues AP, Mota F, Durao M, Frutuoso C, Amaral N, de Oliveira CF. Neoadjuvant chemotherapy in advanced vulvar cancer. Int J Gynecol Cancer. 2010; 20:294–298.32. Aragona AM, Cuneo N, Soderini AH, Alcoba E, Greco A, Reyes C, et al. Tailoring the treatment of locally advanced squamous cell carcinoma of the vulva: neoadjuvant chemotherapy followed by radical surgery: results from a multicenter study. Int J Gynecol Cancer. 2012; 22:1258–1263.33. Han SN, Vergote I, Amant F. Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. Int J Gynecol Cancer. 2012; 22:865–868.34. De Andres L, Brunet J, Lopez-Pousa A, Burgues J, Vega M, Tabernero JM, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin Oncol. 1995; 13:1493–1500.35. Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Jpn J Clin Oncol. 2010; 40:90–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Neoadjuvant chemotherapy with 5-fluorouracial infusion and cisplatin for locally advanced, untreated squamous cell carcinoma of the head and neck
- A Case of Squamous Cell Carcinoma of The Ovary Showing Complete Remission to Combination Chemotherapy Composed of Paclitaxel and Cisplatin
- Efficacy and tolerability of paclitaxel, ifosfamide, and cisplatin as a neoadjuvant chemotherapy in locally advanced cervical carcinoma
- Chemotherapy in Advanced Urothelial Carcinoma