Yonsei Med J.
2013 Nov;54(6):1394-1399. 10.3349/ymj.2013.54.6.1394.
Relationships of Coagulation Factor XIII Activity with Cell-Type and Stage of Non-Small Cell Lung Cancer
- Affiliations
-
- 1Division of Pulmonary, Sleep and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. chepraxis@korea.ac.kr
- 2Department of Clinical Pathology, College of Medicine, Kangwon National University, Chuncheon, Korea.
- 3Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 4Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 1798135
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1394
Abstract
- PURPOSE
Factor XIII (FXIII), a thrombin-activated plasma transglutaminase zymogen, is involved in cancer development and progression through a triggered coagulation pathway. The aim of this study was to examine whether FXIII activity levels differed in non-small cell lung cancer (NSCLC) patients according to histological types and TNM stage when compared with healthy subjects.
MATERIALS AND METHODS
Twenty-eight NSCLC patients and 28 normal controls who had been individually age-, gender-, body mass index-, smoking status-, and smoking amount-matched were enrolled: 13 adenocarcinomas, 11 squamous cell carcinomas, and four undifferentiated NSCLCs; four stage I, two stage II, 12 stage III, and 10 stage IV NSCLCs. FXIII activity was measured using fluorescence-based protein arrays.
RESULTS
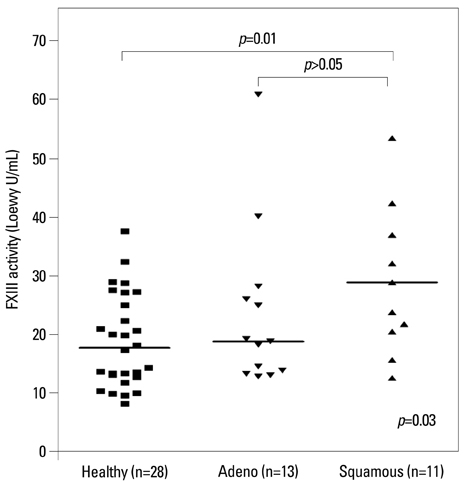
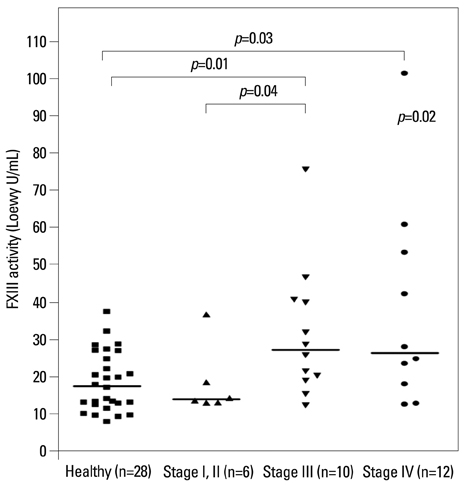
The median FXIII activity level of the NSCLC group [24.2 Loewy U/mL, interquartile range (IQR) 14.9-40.4 Loewy U/mL] was significantly higher than that of the healthy group (17.5 Loewy U/mL, IQR 12.6-26.4 Loewy U/mL) (p=0.01). There were no differences in FXIII activity between adenocarcinoma (median 18.6 Loewy U/mL) and squamous cell carcinoma (median 28.7 Loewy U/mL). NSCLC stage significantly influenced FXIII activity (p=0.02). The FXIII activity of patients with stage III NSCLC (median 27.3 Loewy U/mL, IQR 19.3-40.5 Loewy U/mL) was significantly higher than those of patients with stage I or II (median 14.0 Loewy U/mL, IQR 13.1-23.1 Loewy U/mL, p=0.04). FXIII activity was negatively correlated with aPTT in NSCLC patients (r=-0.38, p=0.04).
CONCLUSION
Patients with advanced-stage NSCLC exhibited higher coagulation FXIII activity than healthy controls and early-stage NSCLC patients.
MeSH Terms
Figure
Reference
-
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–2150.
Article2. Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995; 75:1 Suppl. 191–202.
Article3. Pastorino U. Lung cancer screening. Br J Cancer. 2010; 102:1681–1686.
Article4. Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009; 10:1001–1010.
Article5. Montuenga LM, Pio R. Current challenges in lung cancer early detection biomarkers. Eur J Cancer. 2009; 45:Suppl 1. 377–378.
Article6. Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005; 3:25.
Article7. Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004; 22:1944–1948.
Article8. Sampson MT, Kakkar AK. Coagulation proteases and human cancer. Biochem Soc Trans. 2002; 30:201–207.
Article9. Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004; 104:397–401.
Article10. Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000; 96:3302–3309.
Article11. Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost. 2006; 4:19–25.
Article12. Katona E, Nagy B, Kappelmayer J, Baktai G, Kovács L, Márialigeti T, et al. Factor XIII in bronchoalveolar lavage fluid from children with chronic bronchoalveolar inflammation. J Thromb Haemost. 2005; 3:1407–1413.
Article13. Labat-Robert J. Fibronectin in malignancy. Semin Cancer Biol. 2002; 12:187–195.
Article14. Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002; 62:6966–6972.15. Jones JM, McGonigle NC, McAnespie M, Cran GW, Graham AN. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006; 53:97–101.
Article16. Vairaktaris E, Vassiliou S, Yapijakis C, Spyridonidou S, Vylliotis A, Derka S, et al. Increased risk for oral cancer is associated with coagulation factor XIII but not with factor XII. Oncol Rep. 2007; 18:1537–1543.
Article17. An Y, Bekesova S, Edwards N, Goldman R. Peptides in low molecular weight fraction of serum associated with hepatocellular carcinoma. Dis Markers. 2010; 29:11–20.
Article18. Kiss F, Hevessy Z, Veszprémi A, Katona E, Kiss C, Vereb G, et al. Leukemic lymphoblasts, a novel expression site of coagulation factor XIII subunit A. Thromb Haemost. 2006; 96:176–182.
Article19. Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009; 15:4–9.20. Kim SJ, Suk MH, Choi HM, Kimm KC, Jung KH, Lee SY, et al. The local prevalence of COPD by post-bronchodilator GOLD criteria in Korea. Int J Tuberc Lung Dis. 2006; 10:1393–1398.21. Kwon MH, Kong DH, Jung SH, Suh IB, Kim YM, Ha KS. Rapid determination of blood coagulation factor XIII activity using protein arrays for serodiagnosis of human plasma. Anal Chem. 2011; 83:2317–2323.
Article22. Palumbo JS, Barney KA, Blevins EA, Shaw MA, Mishra A, Flick MJ, et al. Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. J Thromb Haemost. 2008; 6:812–819.
Article23. Bobek V, Kovarík J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004; 58:213–219.
Article24. Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J. 2001; 17:667–673.
Article25. Gouin-Thibault I, Samama MM. Laboratory diagnosis of the thrombophilic state in cancer patients. Semin Thromb Hemost. 1999; 25:167–172.
Article26. Adány R, Bárdos H. Factor XIII subunit A as an intracellular transglutaminase. Cell Mol Life Sci. 2003; 60:1049–1060.
Article27. Lorand L. Factor XIII and the clotting of fibrinogen: from basic research to medicine. J Thromb Haemost. 2005; 3:1337–1348.
Article28. Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost. 2001; 86:57–65.
Article29. Bárdos H, Juhász A, Répássy G, Adány R. Fibrin deposition in squamous cell carcinomas of the larynx and hypopharynx. Thromb Haemost. 1998; 80:767–772.
Article30. Roselli M, Mineo TC, Basili S, Mariotti S, Martini F, Bellotti A, et al. Vascular endothelial growth factor (VEGF-A) plasma levels in non-small cell lung cancer: relationship with coagulation and platelet activation markers. Thromb Haemost. 2003; 89:177–184.
Article31. Jiang WG, Ablin R, Douglas-Jones A, Mansel RE. Expression of transglutaminases in human breast cancer and their possible clinical significance. Oncol Rep. 2003; 10:2039–2044.
Article32. Born P, Lippl F, Ulm K, Gerein P, Lersch C, Eckel F, et al. Reduced levels of coagulation factor XIII in patients with advanced tumor disease. Hepatogastroenterology. 2000; 47:194–198.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Plasma Coagulation Factor XIII Deficiency on the Healing Following Trabeculectomy in Rabbits
- Identification of Plasma Coagulation Factor XIII, Transglutaminase 3 and N epsilon-(gamma-glutamyl) lysine cross-Link in the Silicotic Nodule by Immunohistochemistry
- A Study on Lung Function in Patients with Bronchogenic Cancer
- Cutaneous Metastasis from Small Cell Lung Cancer Expressing Thyroid Transcription Factor-1
- Clinical Significant of S-Phase Fraction in Small Lung Cancer