J Korean Assoc Oral Maxillofac Surg.
2015 Jun;41(3):119-123. 10.5125/jkaoms.2015.41.3.119.
Effects of fresh mineralized dentin and cementum on socket healing: a preliminary study in dogs
- Affiliations
-
- 1Dental Research Center, Department of Periodontics, Dental School, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Endodontic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3Dental School, International Branch of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 4Iran Veterinary Organization, Tehran, Iran.
- 5Department of Anatomical Sciences, Medicine Faculty, North Khorasan University of Medical Sciences, Bojnurd, Iran. shahahmadpour@gmail.com
- KMID: 1797840
- DOI: http://doi.org/10.5125/jkaoms.2015.41.3.119
Abstract
OBJECTIVES
Dentin is composed of many minerals and growth factors. Based on this composition, we studied its effect as a possible regenerative material for alveolar healing.
MATERIALS AND METHODS
This study was conducted using four 2.5-year-old mongrel dogs (male; weight, 25 to 30 kg). The third mandibular premolars were carefully mobilized with a dental elevator and then removed using forceps. The crown portions of the extracted teeth were removed with cutters, and the root portions of the remaining teeth were collectively trimmed as closely as possible to 350 to 500 microm. Dentin and cementum (DC) chips harvested from the extracted teeth were soaked in blood and packed into the fresh sockets (autograft). Biopsies were performed at the ends of day 14 and day 56 following implantation. Data were expressed as mean+/-standard deviation and compared with t-test results.
RESULTS
The ratio of SA(bone) to total area of each probe was determined and was 170+/-16 microm2 for the control group and 71+/-14 microm2 for the DC group, a significant difference (P<0.05).
CONCLUSION
DC particulate grafts offered no improvement in bone regeneration in alveolar extraction sockets.
Keyword
MeSH Terms
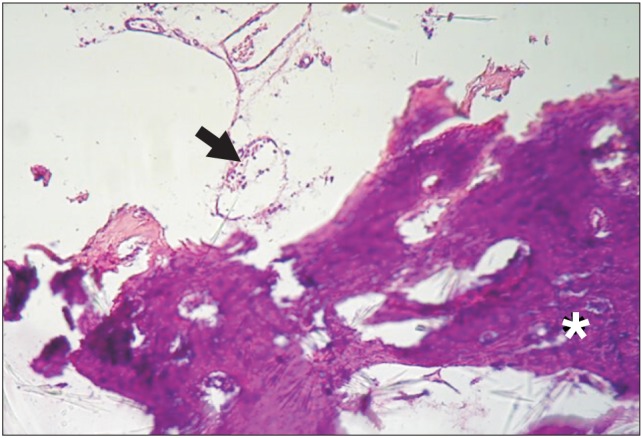
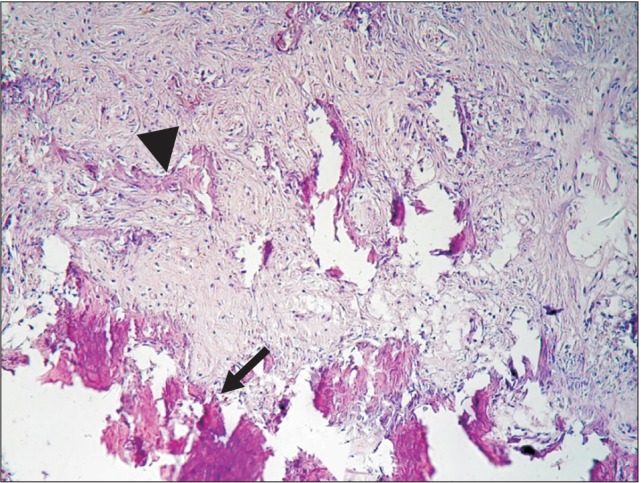
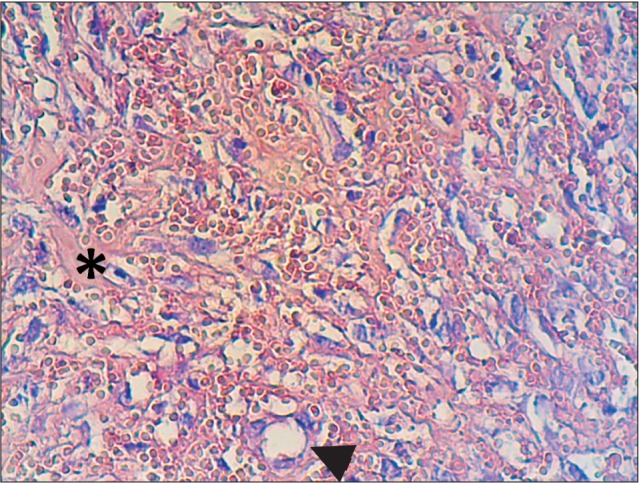
Figure
Cited by 1 articles
-
Discussion: Effects of fresh mineralized dentin and cementum on socket healing: a preliminary study in dogs
In-Woong Um, Woo-Jin Cho
J Korean Assoc Oral Maxillofac Surg. 2015;41(3):124-124. doi: 10.5125/jkaoms.2015.41.3.124.
Reference
-
1. Irinakis T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J Can Dent Assoc. 2006; 72:917–922. PMID: 17187706.2. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004; 31:820–828. PMID: 15367183.
Article3. Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012; 23(Suppl 5):1–21. PMID: 22211303.
Article4. Dimova C. Socket preservation procedure after tooth extraction. Key Engineering Materials. 2014; 587:325–330.
Article5. Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012; 23(Suppl 5):22–38. PMID: 22211304.
Article6. Sattayasanskul W, Brook IM, Lamb DJ. Dense hydroxyapatite root replica implantation: measurement of mandibular ridge preservation. Int J Oral Maxillofac Implants. 1988; 3:203–207. PMID: 3248855.7. Jung RE, Siegenthaler DW, Hämmerle CH. Postextraction tissue management: a soft tissue punch technique. Int J Periodontics Restorative Dent. 2004; 24:545–553. PMID: 15626317.8. Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011; 11:7442–7445. PMID: 22103215.
Article9. Huggins C, Wiseman S, Reddi AH. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med. 1970; 132:1250–1258. PMID: 4929179.
Article10. Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990; 5:717–723. PMID: 2396498.11. Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 1967; 94:781–789. PMID: 4226076.
Article12. Bessho K, Tagawa T, Murata M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J Oral Maxillofac Surg. 1990; 48:162–169. PMID: 2299457.
Article13. Butler WT, Mikulski A, Urist MR, Bridges G, Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977; 56:228–232. PMID: 265954.
Article14. Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010; 118:438–441.
Article15. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967; 12:999–1008. PMID: 4226721.
Article16. Murata M. Bone engineering using human demineralized dentin matrix and recombinant human BMP2. J Hard Tissue Biol. 2005; 14:80–81.
Article17. Reed MG, Howard CV, de Yanés GS. One-stop stereology: the estimation of 3D parameters using isotropic rulers. J Microsc. 2010; 239:54–65. PMID: 20579269.
Article18. Wang X, Zauel RR, Rao DS, Fyhrie DP. Cancellous bone lamellae strongly affect microcrack propagation and apparent mechanical properties: separation of patients with osteoporotic fracture from normal controls using a 2D nonlinear finite element method (biomechanical stereology). Bone. 2008; 42:1184–1192. PMID: 18378204.
Article19. Lewandrowski KU, Tomford WW, Schomacker KT, Deutsch TF, Mankin HJ. Improved osteoinduction of cortical bone allografts: a study of the effects of laser perforation and partial demineralization. J Orthop Res. 1997; 15:748–756. PMID: 9420606.
Article20. Reddi AH. Bone matrix in the solid state: geometric influence on differentiation of fibroblasts. Adv Biol Med Phys. 1974; 15:1–18. PMID: 4600893.
Article21. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503. PMID: 20060336.
Article22. Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010; 81:1264–1272. PMID: 20476887.
Article23. Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011; 37:375–379.
Article24. Devecioğlu D, Tözüm TF, Sengün D, Nohutcu RM. Biomaterials in periodontal regenerative surgery: effects of cryopreserved bone, commercially available coral, demineralized freeze-dried dentin, and cementum on periodontal ligament fibroblasts and osteoblasts. J Biomater Appl. 2004; 19:107–120. PMID: 15381784.25. Gomes MF, Destro MF, Banzi EC, Vieira EM, Morosolli AR, Goulart Md. Optical density of bone repair after implantation of homogenous demineralized dentin matrix in diabetic rabbits. Braz Oral Res. 2008; 22:275–280. PMID: 18949316.
Article26. Urist MR, Granstein R, Nogami H, Svenson L, Murphy R. Transmembrane bone morphogenesis across multiple-walled diffusion chambers. New evidence for a diffusible bone morphogenetic property. Arch Surg. 1977; 112:612–619. PMID: 857763.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Discussion: Effects of fresh mineralized dentin and cementum on socket healing: a preliminary study in dogs
- Histology of dental pulp healing after tooth replantation in rats
- Histological characteristics of newly formed cementum in surgically created one-wall intrabony defects in a canine model
- Collagen biology for bone regenerative surgery
- Study of bone healing pattern in extraction socket after application of demineralized dentin matrix material