J Korean Med Sci.
2009 Feb;24(1):104-109. 10.3346/jkms.2009.24.1.104.
Tissue Engineering of Injectable Soft tissue Filler: Using Adipose Stem Cells and Micronized Acellular Dermal Matrix
- Affiliations
-
- 1Department of Plastic Surgery, the Catholic University of Korea, Seoul, Korea. prsdrlim@yahoo.com
- KMID: 1794414
- DOI: http://doi.org/10.3346/jkms.2009.24.1.104
Abstract
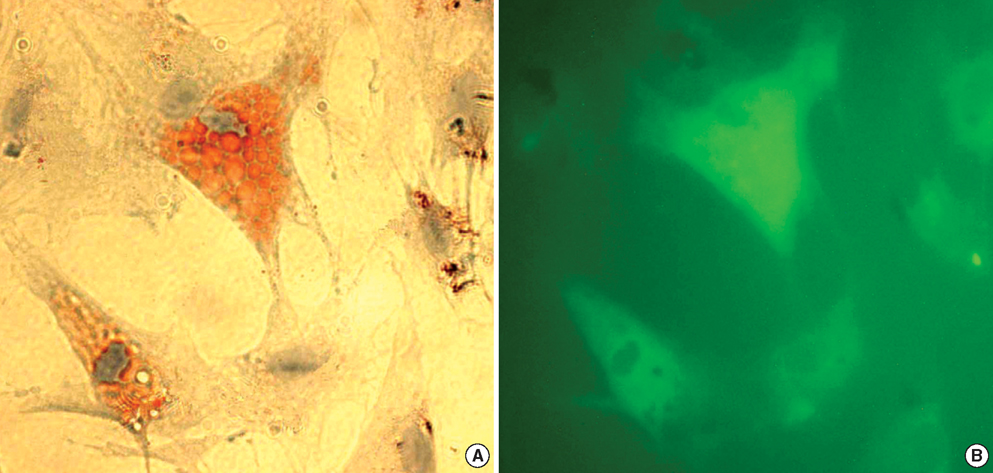
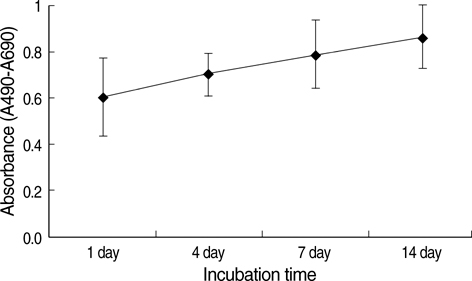
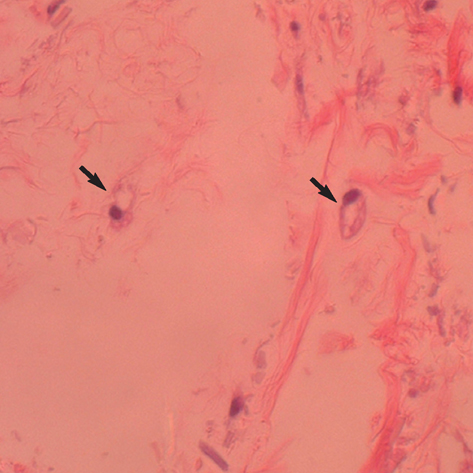
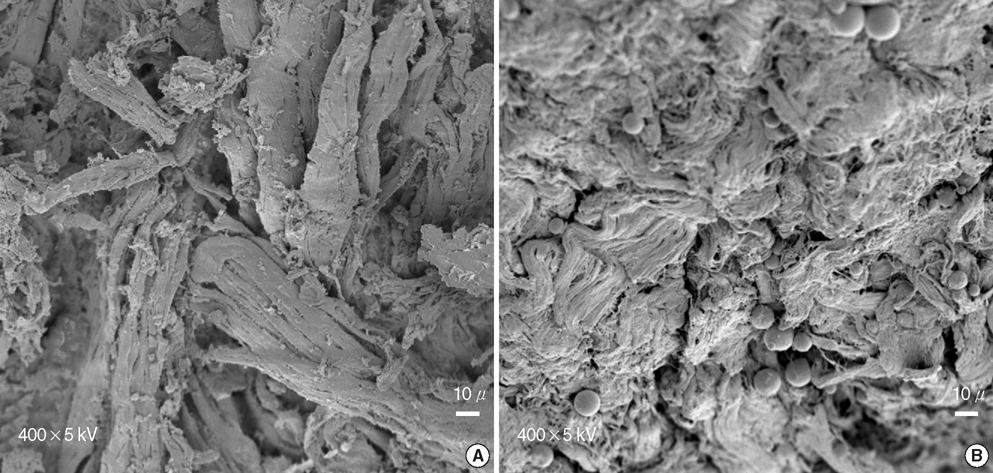
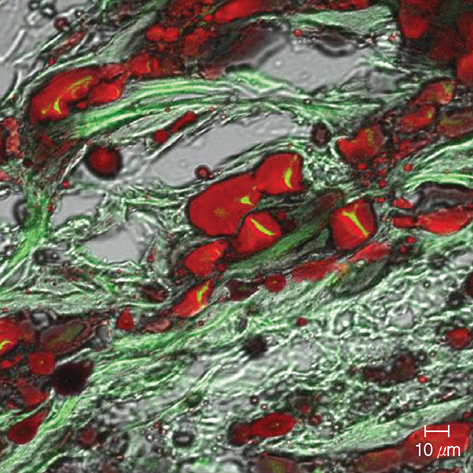
- In this study of a developed soft tissue filler, adipose tissue equivalents were constructed using adipose stem cells (ASCs) and micronized acellular dermal matrix (Alloderm). After labeling cultured human ASCs with fluorescent green protein and attaching them to micronized Alloderm (5X10(5) cells/1 mg), ASC-Alloderm complexes were cultured in adipogenic differentiation media for 14 days and then injected into the dorsal cranial region of nude male mice. The viabilities of ASCs in micronized Alloderm were determined at 1, 4, 7, and 14 days, and complexes, which had been cultured for 14 days and implanted in vivo for 2 months, were histologically evaluated by light, confocal, and scanning electron microscopy. The viabilities represented that ASCs in micronized Alloderm were alive during the culture period. ASC-Alloderm complexes cultured for 14 days contained round cells with large lipid vesicles by light microscopy and many spherical cells by SEM. ASCs in implanted ASCAlloderm complexes harvested from mice at 2 months postinjection were histologically found to have differentiated into adipocytes which had green fluorescence dye. Micronized Alloderm may be found useful as scaffold for human ASCs when constructing fat tissue for three-dimensional soft tissue filling. The present study suggests that ASC-Alloderm complexes can be used as injectable three-dimensional soft tissue fillers.
Keyword
MeSH Terms
-
Adipocytes/*cytology
Adipogenesis
Adipose Tissue/cytology
Animals
Cell Differentiation
Cells, Cultured
Collagen/*chemistry
Fluorescent Dyes/chemistry
Injections, Subcutaneous
Male
Mice
Mice, Nude
Microscopy, Electron, Scanning
Stem Cell Transplantation/*methods
Stem Cells/cytology/pathology
Time Factors
Tissue Engineering/*methods
Transplantation, Heterologous
Figure
Reference
-
1. Maloney BP, Murphy BA, Cole HP 3rd. Cymetra. Facial Plast Surg. 2004. 20:129–134.
Article2. Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri EZ, Dani C, Ailhaud G. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004. 315:255–263.
Article3. Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006. 183:133–140.
Article4. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article5. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006. 24:150–154.
Article6. Billings E Jr, May JW Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989. 83:368–381.
Article7. Patrick CW Jr. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001. 263:361–366.
Article8. De Ugarte DA, Ashjian PH, Elbarbary A, Hedrick MH. Future of fat as raw material for tissue regeneration. Ann Plast Surg. 2003. 50:215–219.
Article9. von Heimburg D, Hemmrich K, Zachariah S, Staiger H, Pallua N. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respir Physiol Neurobiol. 2005. 146:107–116.10. Cho SW, Kim SS, Rhie JW, Cho HM, Choi CY, Kim BS. Engineering of volume-stable adipose tissues. Biomaterials. 2005. 26:3577–3585.
Article11. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article12. Hickerson WL, Compton C, Fletchall S, Smith LR. Cultured epidermal autografts and allodermis combination for permanent burn wound coverage. Burns. 1994. 20:Suppl 1. S52–S55.
Article13. Izumi K, Takacs G, Terashi H, Feinberg SE. Ex vivo development of a composite human oral mucosal equivalent. J Oral Maxillofac Surg. 1999. 57:571–577.
Article14. Izumi K, Terashi H, Marcelo CL, Feinberg SE. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J Dent Res. 2000. 79:798–805.
Article15. Patrick CW Jr, Chauvin PB, Hobley J, Reece GP. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999. 5:139–151.
Article16. Halbleib M, Skurk T, de Luca C, von Heimburg D, Hauner H. Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I: in vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials. 2003. 24:3125–3132.
Article17. Schoeller T, Lille S, Wechselberger G, Otto A, Mowlavi A, Piza-Katzer H, Mowlawi A. Histomorphologic and volumetric analysis of implanted autologous preadipocyte cultures suspended in fibrin glue: a potential new source for tissue augmentation. Aesthetic Plast Surg. 2001. 25:57–63.
Article18. Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998. 95:1062–1066.
Article19. Yoo G, Yea BH, Rhie JW, Kwon H, Wee SS, Ahn ST. Growth and Differentiation of Preadipocytes in Alginate and Collagen Gels. J Korean Soc Plast Reconstr Surg. 2000. 27:386–392.20. Cheng JT, Perkins SW, Hamilton MM. Collagen and injectable fillers. Otolaryngol Clin North Am. 2002. 35:73–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Avidin and Biotin in Attachment of Human Adipose Stem Cells to Micronized Acellular Dermal Matrix
- Injectable Tissue-Engineered Soft Tissue for Tissue Augmentation
- Treatment of Linear Morphea (en Coup de Sabre) with Micronized Acellular Dermal Matrix Filler: A Case Report
- Periodontal tissue engineering by hPDLF seeding on scaffold
- Use of Acellular Dermal Matrix in Reconstructive Surgery: A Review