J Korean Med Sci.
2013 Aug;28(8):1248-1252. 10.3346/jkms.2013.28.8.1248.
Imatinib Plasma Monitoring-Guided Dose Modification for Managing Imatinib-Related Toxicities in Gastrointestinal Stromal Tumor Patients
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykkang@amc.seoul.kr
- KMID: 1793034
- DOI: http://doi.org/10.3346/jkms.2013.28.8.1248
Abstract
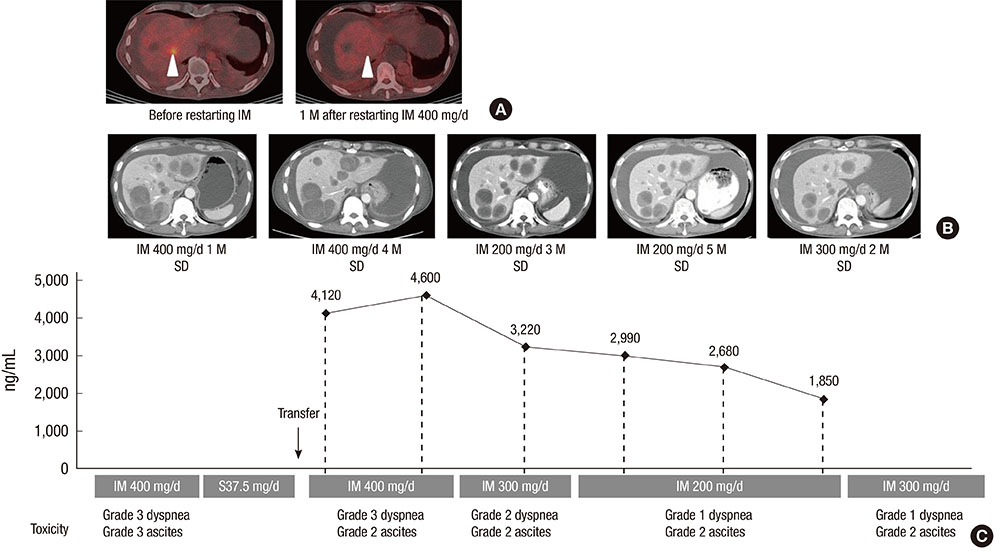
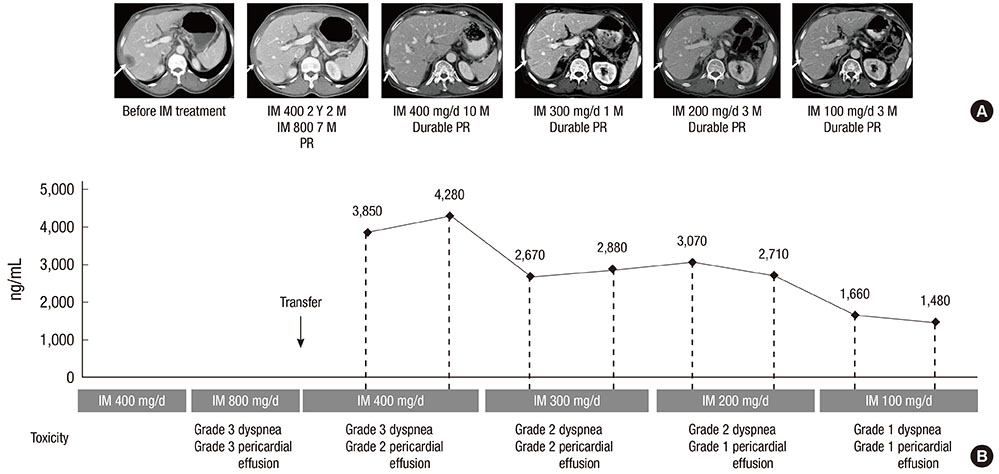
- Imatinib, the first-line treatment in patients with advanced gastrointestinal stromal tumors (GIST), is generally well tolerated, although some patients have difficulty tolerating the standard dose of 400 mg/day. Adjusting imatinib dosage by plasma level monitoring may facilitate management of patients who experience intolerable toxicities due to overexposure to the drug. We present two cases of advanced GIST patients in whom we managed imatinib-related toxicities through dose modifications guided by imatinib plasma level monitoring. Imatinib blood level testing may be a promising approach for fine-tuning imatinib dosage for better tolerability and optimal clinical outcomes in patients with advanced GIST.
Keyword
MeSH Terms
-
Aged
Antineoplastic Agents/blood/*therapeutic use
Benzamides/blood/*therapeutic use
Drug Monitoring
Exons
Gastrointestinal Neoplasms/*drug therapy/pathology/radiography
Gastrointestinal Stromal Tumors/*drug therapy/pathology/radiography
Humans
Liver Neoplasms/secondary
Male
Mutation
Piperazines/blood/*therapeutic use
Positron-Emission Tomography
Proto-Oncogene Proteins c-kit/genetics
Pyrimidines/blood/*therapeutic use
Tomography, X-Ray Computed
Antineoplastic Agents
Benzamides
Piperazines
Proto-Oncogene Proteins c-kit
Pyrimidines
Figure
Cited by 1 articles
-
Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor
Dong-Hoe Koo, Min-Hee Ryu, Kyoung-Mee Kim, Han-Kwang Yang, Akira Sawaki, Seiichi Hirota, Jie Zheng, Bo Zhang, Chin-Yuan Tzen, Chun-Nan Yeh, Toshirou Nishida, Lin Shen, Li-Tzong Chen, Yoon-Koo Kang
Cancer Res Treat. 2016;48(4):1155-1166. doi: 10.4143/crt.2016.187.
Reference
-
1. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.2. Ryu MH, Kang WK, Bang YJ, Lee KH, Shin DB, Ryoo BY, Roh JK, Kang JH, Lee H, Kim TW, et al. A prospective, multicenter, phase 2 study of imatinib mesylate in korean patients with metastatic or unresectable gastrointestinal stromal tumor. Oncology. 2009; 76:326–332.3. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009; 27:3141–3147.4. Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004; 22:935–942.5. Yoo C, Ryu MH, Kang BW, Yoon SK, Ryoo BY, Chang HM, Lee JL, Beck MY, Kim TW, Kang YK. Cross-sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: impact of gastrointestinal resection on exposure to imatinib. J Clin Oncol. 2010; 28:1554–1559.6. Widmer N, Decosterd LA, Leyvraz S, Duchosal MA, Rosselet A, Debiec-Rychter M, Csajka C, Biollaz J, Buclin T. Relationship of imatinib-free plasma levels and target genotype with efficacy and tolerability. Br J Cancer. 2008; 98:1633–1640.7. George S, Trent JC. The role of imatinib plasma level testing in gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 2011; 67:S45–S50.8. Ryu MH, Lee JL, Chang HM, Kim TW, Kang HJ, Sohn HJ, Lee JS, Kang YK. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn J Clin Oncol. 2006; 36:17–24.9. Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006; 42:1093–1103.10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010; 28:1247–1253.11. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993; 217:72–77.12. Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005; 44:879–894.13. Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y. IRIS (International Randomized Interferon vs STI571) Study Group. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS Study. Blood. 2008; 111:4022–4028.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor
- A Case of Generalized Keratosis Pilaris Induced by Imatinib Mesylate
- Imatinib-induced hepatitis treated by corticosteroids in a patient with metastatic gastrointestinal stromal tumor
- Diagnosis and Treatment of Imatinib-induced Pneumonitis in a Patient with High-risk Rectal Gastrointestinal Stromal Tumor
- A Case of Disseminated Intra-abdominal Gastrointestinal Stromal Tumor Managed with Low Dose Imatinib