J Korean Med Sci.
2013 Aug;28(8):1233-1237. 10.3346/jkms.2013.28.8.1233.
Interfractional Variation of Radiation Target and Adaptive Radiotherapy for Totally Resected Glioblastoma
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dh8.lim@samsung.com
- KMID: 1793031
- DOI: http://doi.org/10.3346/jkms.2013.28.8.1233
Abstract
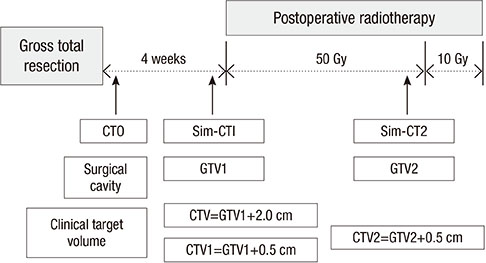
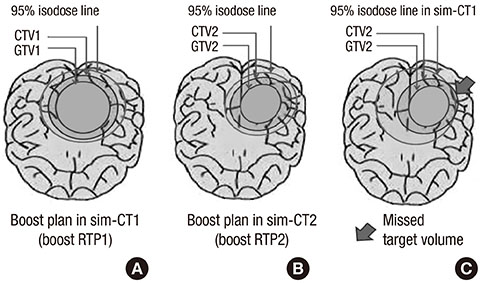
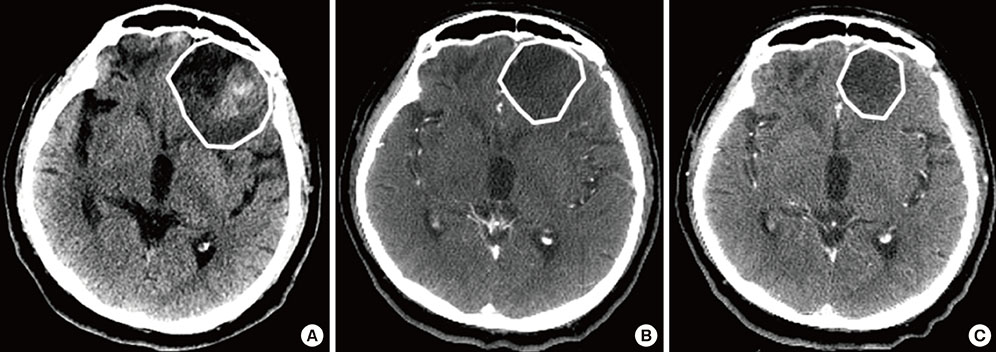
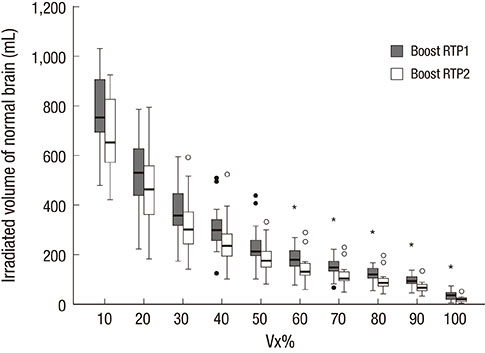
- This study aimed to evaluate the effects of volume adapted re-planning for radiotherapy (RT) after gross total resection (GTR) for glioblastoma. Nineteen patients with glioblastoma who underwent GTR and postoperative RT were analyzed. The volumes of the surgical cavity on computed tomography (CT) obtained one day after GTR (CT0), the first RT simulation CT (sim-CT1), and the second simulation CT for the boost RT plan (sim-CT2) were compared. The boost RT plan was based on the surgical cavity observed on the sim-CT2 (boost RTP2) and was compared with that based on the surgical cavity observed on the sim-CT1 (boost RTP1). The volume reduction ratios were 14.4%-51.3% (median, 29.0%) between CT0 and sim-CT1 and -7.9%-71.9% (median, 34.9%) between sim-CT1 and sim-CT2 (P < 0.001). The normal brain volumes in boost RTP1 were significantly reduced in boost RTP2, especially at high dose levels. Target volume in sim-CT2 which was not covered with the boost RTP1, developed in five cases (26.3%). The surgical cavity volume was reduced following surgery in patients with glioblastoma who underwent GTR. The application of volume-adapted re-planning during RT could decrease the irradiated volume of normal brain and prevent a target miss for boost RT.
Keyword
MeSH Terms
Figure
Reference
-
1. Seither RB, Jose B, Paris KJ, Lindberg RD, Spanos WJ. Results of irradiation in patients with high-grade gliomas evaluated by magnetic resonance imaging. Am J Clin Oncol. 1995; 18:297–299.2. Shapiro WR, Green SB, Burger PC, Mahaley MS Jr, Selker RG, VanGilder JC, Robertson JT, Ransohoff J, Mealey J Jr, Strike TA, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma: Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989; 71:1–9.3. Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M. 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol. 1999; 53:53–57.4. Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989; 16:1405–1409.5. Nelson DF, Curran WJ Jr, Scott C, Nelson JS, Weinstein AS, Ahmad K, Constine LS, Murray K, Powlis WD, Mohiuddin M, et al. Hyperfractionated radiation therapy and bis-chlorethyl nitrosourea in the treatment of malignant glioma: possible advantage observed at 72.0 Gy in 1.2 Gy B.I.D. fractions: report of the Radiation Therapy Oncology Group Protocol 8302. Int J Radiat Oncol Biol Phys. 1993; 25:193–207.6. Jansen EP, Dewit LG, van Herk M, Bartelink H. Target volumes in radiotherapy for high-grade malignant glioma of the brain. Radiother Oncol. 2000; 56:151–156.7. Chang EL, Akyurek S, Avalos T, Rebueno N, Spicer C, Garcia J, Famiglietti R, Allen PK, Chao KS, Mahajan A, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007; 68:144–150.8. Giraud P, Kantor G, Loiseau H, Rosenzweig KE. Target definition in the thorax and central nervous system. Semin Radiat Oncol. 2005; 15:146–156.9. Halperin EC, Bentel G, Heinz ER, Burger PC. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989; 17:1347–1350.10. Burger PC, Dubois PJ, Schold SC Jr, Smith KR Jr, Odom GL, Crafts DC, Giangaspero F. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983; 58:159–169.11. Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology. 1989; 170:211–217.12. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987; 66:865–874.13. Park I, Tamai G, Lee MC, Chuang CF, Chang SM, Berger MS, Nelson SJ, Pirzkall A. Patterns of recurrence analysis in newly diagnosed glioblastoma multiforme after three-dimensional conformal radiation therapy with respect to pre-radiation therapy magnetic resonance spectroscopic findings. Int J Radiat Oncol Biol Phys. 2007; 69:381–389.14. Datta NR, David R, Gupta RK, Lal P. Implications of contrast-enhanced CT-based and MRI-based target volume delineations in radiotherapy treatment planning for brain tumors. J Cancer Res Ther. 2008; 4:9–13.15. Manon R, Hui S, Chinnaiyan P, Suh J, Chang E, Timmerman R, Phan S, Das R, Mehta M. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol Cancer Res Treat. 2004; 3:303–307.16. Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981; 7:243–252.17. Constine LS, Konski A, Ekholm S, McDonald S, Rubin P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988; 15:319–330.18. Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010; 76:S20–S27.19. Maire JP, Coudin B, Guérin J, Caudry M. Neuropsychologic impairment in adults with brain tumors. Am J Clin Oncol. 1987; 10:156–162.20. Hochberg FH, Slotnick B. Neuropsychologic impairment in astrocytoma survivors. Neurology. 1980; 30:172–177.21. Merchant TE, Kiehna EN, Li C, Shukla H, Sengupta S, Xiong X, Gajjar A, Mulhern RK. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006; 65:210–221.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dosimetric Effects of Air Pocket during Magnetic Resonance-Guided Adaptive Radiation Therapy for Pancreatic Cancer
- Limited field adaptive radiotherapy for glioblastoma: changes in target volume and organ at risk doses
- Radiotherapy Results of Malignant Astrocytoma and Glioblastoma Multiforme
- Inhibition of the Spectraplakin Protein Microtubule ActinCrosslinking Factor 1 Sensitizes Glioblastomas to Radiation
- Feasibility of artificial intelligence-driven interfractional monitoring of organ changes by mega-voltage computed tomography in intensity-modulated radiotherapy of prostate cancer