J Korean Med Sci.
2010 Dec;25(12):1784-1791. 10.3346/jkms.2010.25.12.1784.
Effect of Probiotic Lactobacillus (Lacidofil(R) Cap) for the Prevention of Antibiotic-associated Diarrhea: A Prospective, Randomized, Double-blind, Multicenter Study
- Affiliations
-
- 1Department of Internal Medicine and Preventive Medicine, Ewha Womans University School of Medicine, Seoul, Korea. jassa@ewha.ac.kr
- 2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 6Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Ajou University College of Medicine, Suwon, Korea.
- 8Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 9Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
- 10Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea.
- KMID: 1792904
- DOI: http://doi.org/10.3346/jkms.2010.25.12.1784
Abstract
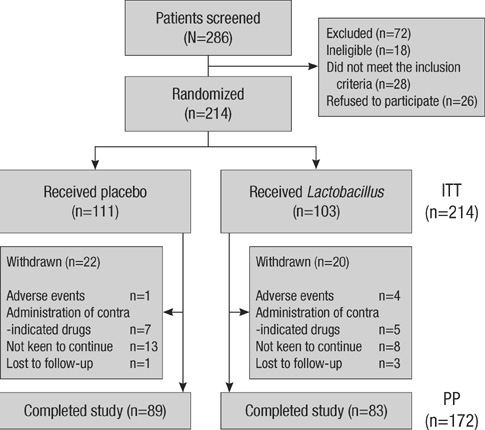
- Antibiotic-associated diarrhea (AAD) is a common complication of antibiotic use. There is growing interest in probiotics for the treatment of AAD and Clostridium difficile infection because of the wide availability of probiotics. The aim of this multicenter, randomized, placebo-controlled, double-blind trial was to assess the efficacy of probiotic Lactobacillus (Lacidofil(R) cap) for the prevention of AAD in adults. From September 2008 to November 2009, a total of 214 patients with respiratory tract infection who had begun receiving antibiotics were randomized to receive Lactobacillus (Lacidofil(R) cap) or placebo for 14 days. Patients recorded bowel frequency and stool consistency daily for 14 days. The primary outcome was the proportion of patients who developed AAD within 14 days of enrollment. AAD developed in 4 (3.9%) of 103 patients in the Lactobacillus group and in 8 (7.2%) of 111 patients in the placebo group (P=0.44). However, the Lactobacillus group showed lower change in bowel frequency and consistency (50/103, 48.5%) than the placebo group (35/111, 31.5%) (P=0.01). Although the Lacidofil(R) cap does not reduce the rate of occurrence of AAD in adult patients with respiratory tract infection who have taken antibiotics, the Lactobacillus group maintains their bowel habits to a greater extent than the placebo group.
MeSH Terms
Figure
Cited by 2 articles
-
Antibiotic-Associated Diarrhea in 3 to 6 Month Old Infants with Febrile Urinary Tract Infections
Chong Bock Won, Min Chae Kim, Byung Wook Eun, Yong Han Sun, Kang Ho Cho, Hann Tcha, In Sang Jeon
Korean J Pediatr Infect Dis. 2012;19(1):12-18. doi: 10.14776/kjpid.2012.19.1.12.Antibiotic-Associated Diarrhea in 3 to 6 Month Old Infants with Febrile Urinary Tract Infections
Chong Bock Won, Min Chae Kim, Byung Wook Eun, Yong Han Sun, Kang Ho Cho, Hann Tcha, In Sang Jeon
Korean J Pediatr Infect Dis. 2012;19(1):12-18. doi: 10.14776/kjpid.2012.19.1.12.
Reference
-
1. McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998. 16:292–307.
Article2. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002. 346:334–339.3. Ackermann G, Thomalla S, Ackermann F, Schaumann R, Rodloff AC, Ruf BR. Prevalence and characteristics of bacteria and host factors in an outbreak situation of antibiotic-associated diarrhoea. J Med Microbiol. 2005. 54:149–153.
Article4. McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000. 12:193–207.
Article5. McFarland LV. A review of the evidence of health claims for biotherapeutic agents. Microb Ecol Health Dis. 2000. 12:65–76.
Article6. Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, LaMont JT, Kelly CP. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun. 2001. 69:2762–2765.7. Elmer GW. Probiotics: "Living drugs". Am J Health Syst Pharm. 2001. 58:1101–1109.
Article8. Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease activity. CMAJ. 2004. 171:466–472.9. Katikireddi V. UK launches inquired into Clostridium difficile outbreak. CMAJ. 2005. 173:138.10. McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006. 101:812–822.
Article11. Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002. 97:2744–2749.
Article12. Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999. 104:e64.13. Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999. 135:564–568.
Article14. Szajewska H, Kotowska M, Mrukowicz JZ, Armańska M, Mikołajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001. 138:361–365.
Article15. Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001. 76:883–889.
Article16. Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, Santarelli L, Cammarota G, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion. 2001. 63:1–7.17. Beausoleil M, Fortier N, Guénette S, L'ecuyer A, Savoie M, Franco M, Lachaine J, Weiss K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007. 21:732–736.18. Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007. 335:80.
Article19. Kale-Pradhan PB, Jassal HK, Wilhelm SM. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy. 2010. 30:119–126.20. Szymański H, Armańska M, Kowalska-Duplaga K, Szajewska H. Bifidobacterium longum PL03, Lactobacillus rhamnosus KL53A, and Lactobacillus plantarum PL02 in the prevention of antibiotic-associated diarrhea in children: a randomized controlled pilot trial. Digestion. 2008. 78:13–17.21. Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987. 31:1231–1233.
Article22. Alander M, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett Appl Microbiol. 1997. 24:361–364.
Article23. Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci. 1992. 37:121–128.24. Ling WH, Korpela R, Mykkänen H, Salminen S, Hänninen O. Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. J Nutr. 1994. 124:18–23.
Article25. Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective-study. Gastroenterology. 1989. 96:981–988.26. Coconnier MH, Liévin V, Bernet-Camard MF, Hudault S, Servin AL. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997. 41:1046–1052.
Article27. Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008. 28:154–161.28. De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J. 2005. 24:278–280.
Article29. Young RJ, Vanderhoof JA. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr. 2004. 39:436–437.
Article30. Jirapinyo P, Densupsoontorn N, Thamonsiri N, Wongarn R. Prevention of antibiotic-associated diarrhea in infants by probiotics. J Med Assoc Thai. 2002. 85:Suppl 2. S739–S742.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Probiotics in Children: What Is the Evidence?
- Probiotic Supplementation for Treatment of Helicobacter pylori Infection: A Double-Blind Randomized Clinical Trial
- Efficacy of the Probiotic Probiotical Confirmed in Acute Gastroenteritis
- Risk and Protective Factors for Gastrointestinal Symptoms associated with Antibiotic Treatment in Children: A Population Study
- Effect of Saccharomyces boulardii CNCM-I 3799 and Bacillus subtilis CU-1 on Acute Watery Diarrhea: A Randomized Double-Blind PlaceboControlled Study in Indian Children