J Korean Med Sci.
2013 Mar;28(3):402-408. 10.3346/jkms.2013.28.3.402.
Time-Dependent Expression Patterns of Cardiac Aquaporins Following Myocardial Infarction
- Affiliations
-
- 1Department of Cardiology, Dong-A University College of Medicine, Busan, Korea.
- 2Department of Physiology, Dong-A University College of Medicine, Busan, Korea. hrbae@dau.ac.kr
- KMID: 1786938
- DOI: http://doi.org/10.3346/jkms.2013.28.3.402
Abstract
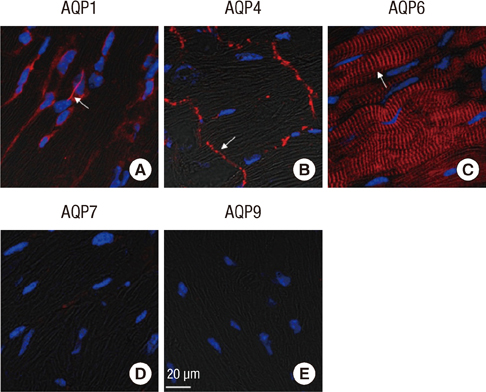
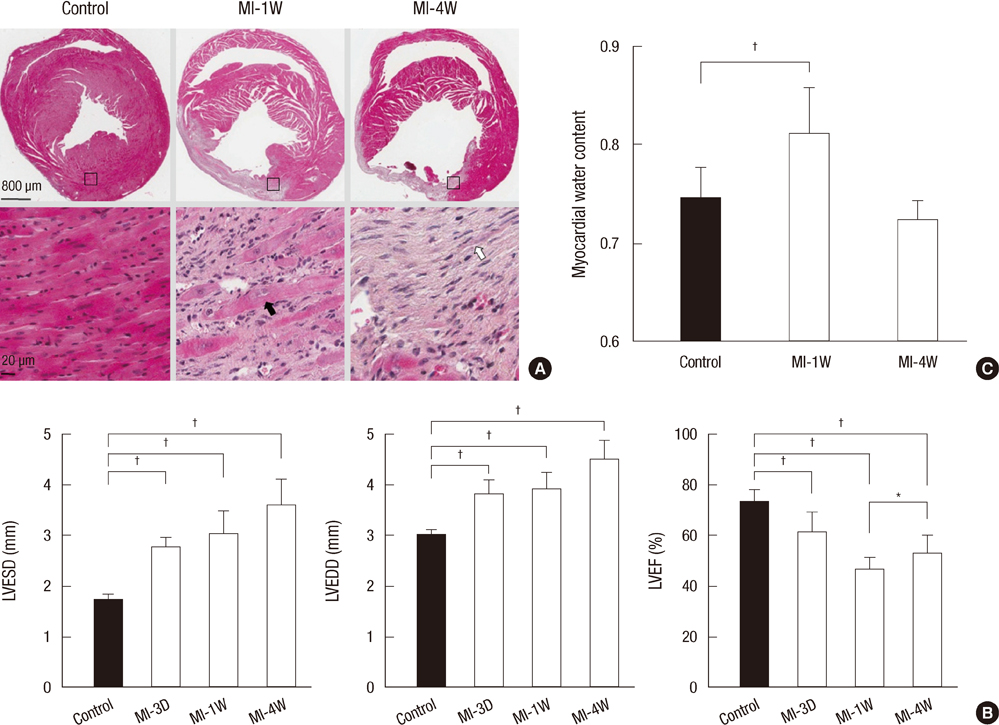
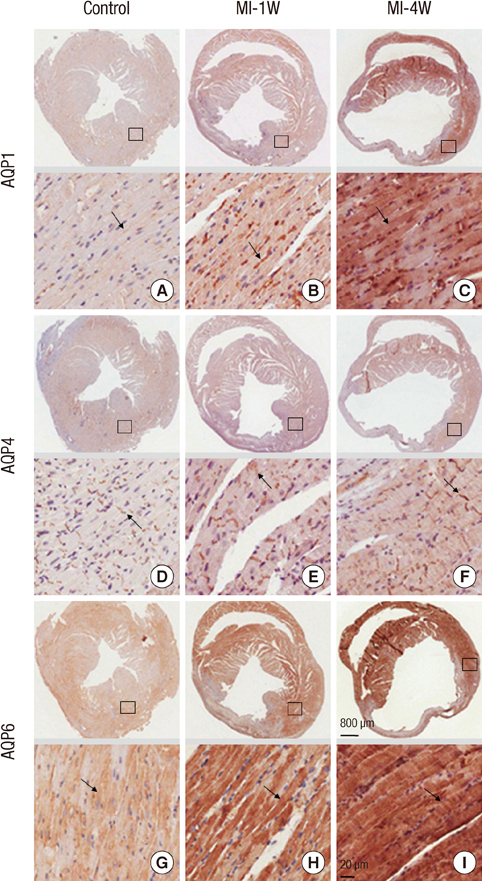
- Aquaporins (AQPs) are expressed in myocardium and the implication of AQPs in myocardial water balance has been suggested. We investigated the expression patterns of AQP subtypes in normal myocardium and their changes in the process of edema formation and cardiac dysfunction following myocardial infarction (MI). Immunostaining demonstrated abundant expression of AQP1, AQP4, and AQP6 in normal mouse heart; AQP1 in blood vessels and cardiac myocytes, AQP4 exclusively on the intercalated discs between cardiac myocytes and AQP6 inside the myocytes. However, neither AQP7 nor AQP9 proteins were expressed in CD1 mouse myocardium. Echocardiography revealed that cardiac function was reduced at 1 week and recovered at 4 weeks after MI, whereas myocardial water content determined by wet-to-dry weight ratio increased at 1 week and rather reduced below the normal at 4 weeks. The expression of cardiac AQPs was up-regulated in MI-induced groups compared with sham-operated control group, but their time-dependent patterns were different. The time course of AQP4 expression coincided with that of myocardial edema and cardiac dysfunction following MI. However, expression of both AQP1 and AQP6 increased persistently up to 4 weeks. Our findings suggest a different role for cardiac AQPs in the formation and reabsorption of myocardial edema after MI.
Keyword
MeSH Terms
-
Animals
Aquaporin 1/metabolism
Aquaporin 4/metabolism
Aquaporin 6/metabolism
Aquaporins/*metabolism
Edema/pathology
Immunohistochemistry
Mice
Muscle Cells/metabolism
Myocardial Infarction/*metabolism/pathology/ultrasonography
Myocardium/metabolism/pathology
Time Factors
Aquaporin 4
Aquaporin 6
Aquaporins
Aquaporin 1
Figure
Reference
-
1. Mehlhorn U, Geissler HJ, Laine GA, Allen SJ. Myocardial fluid balance. Eur J Cardiothorac Surg. 2001. 20:1220–1230.2. Garcia-Dorado D, Andres-Villarreal M, Ruiz-Meana M, Inserte J, Barba I. Myocardial edema: a translational view. J Mol Cell Cardiol. 2012. 52:931–939.3. Bijnens B, Sutherland GR. Myocardial oedema: a forgotten entity essential to the understanding of regional function after ischaemia or reperfusion injury. Heart. 2008. 94:1117–1119.4. Møller JE, Egstrup K, Køber L, Poulsen SH, Nyvad O, Torp-Pedersen C. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J. 2003. 145:147–153.5. St John Sutton M. Quest for diastolic prognostic indicators of clinical outcome after acute myocardial infarction. Circulation. 2008. 117:2570–2572.6. Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006. 63:1386–1392.7. Ishibashi K, Hara S, Kondo S. Aquaporin water channels in mammals. Clin Exp Nephrol. 2009. 13:107–117.8. Verkman AS. Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev Physiol Biochem Pharmacol. 2005. 155:31–55.9. Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000. 6:159–163.10. Butler TL, Au CG, Yang B, Egan JR, Tan YM, Hardeman EC, North KN, Verkman AS, Winlaw DS. Cardiac aquaporin expression in humans, rats, and mice. Am J Physiol Heart Circ Physiol. 2006. 291:H705–H713.11. Egan JR, Butler TL, Au CG, Tan YM, North KN, Winlaw DS. Myocardial water handling and the role of aquaporins. Biochim Biophys Acta. 2006. 1758:1043–1052.12. Ma T, Yang B, Verkman AS. Cloning of a novel water and urea-permeable aquaporin from mouse expressed strongly in colon, placenta, liver, and heart. Biochem Biophys Res Commun. 1997. 240:324–328.13. Nejsum LN, Elkjaer M, Hager H, Frokiaer J, Kwon TH, Nielsen S. Localization of aquaporin-7 in rat and mouse kidney using RT-PCR, immunoblotting, and immunocytochemistry. Biochem Biophys Res Commun. 2000. 277:164–170.14. Liu K, Nagase H, Huang CG, Calamita G, Agre P. Purification and functional characterization of aquaporin-8. Biol Cell. 2006. 98:153–161.15. Au CG, Cooper ST, Lo HP, Compton AG, Yang N, Wintour EM, North KN, Winlaw DS. Expression of aquaporin 1 in human cardiac and skeletal muscle. J Mol Cell Cardiol. 2004. 36:655–662.16. Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009. 19:182–190.17. Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001. 98:14108–14113.18. Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001. 155:113–122.19. Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res. 2011. 108:294–304.20. Shi LB, Skach WR, Ma T, Verkman AS. Distinct biogenesis mechanisms for the water channels MIWC and CHIP28 at the endoplasmic reticulum. Biochemistry. 1995. 34:8250–8256.21. Ohshiro K, Yaoita E, Yoshida Y, Fujinaka H, Matsuki A, Kamiie J, Kovalenko P, Yamamoto T. Expression and immunolocalization of AQP6 in intercalated cells of the rat kidney collecting duct. Arch Histol Cytol. 2001. 64:329–338.22. Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002. 277:39873–39879.23. Jeremic A, Cho WJ, Jena BP. Involvement of water channels in synaptic vesicle swelling. Exp Biol Med (Maywood). 2005. 230:674–680.24. Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991. 324:295–301.25. Nilsson JC, Nielsen G, Groenning BA, Fritz-Hansen T, Sondergaard L, Jensen GB, Larsson HB. Sustained postinfarction myocardial oedema in humans visualised by magnetic resonance imaging. Heart. 2001. 85:639–642.26. Laine GA, Allen SJ. Left ventricular myocardial edema: lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991. 68:1713–1721.27. Warth A, Eckle T, Köhler D, Faigle M, Zug S, Klingel K, Eltzschig HK, Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology. 2007. 107:402–410.28. Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999. 85:1046–1055.29. Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007. 22:778–784.30. Strohschein S, Hüttmann K, Gabriel S, Binder DK, Heinemann U, Steinhäuser C. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia. 2011. 59:973–980.31. Ran X, Wang H, Chen Y, Zeng Z, Zhou Q, Zheng R, Sun J, Wang B, Lv X, Liang Y, et al. Aquaporin-1 expression and angiogenesis in rabbit chronic myocardial ischemia is decreased by acetazolamide. Heart Vessels. 2010. 25:237–247.32. Borok Z, Verkman AS. Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways. J Appl Physiol. 2002. 93:2199–2206.33. Suleymanian MA, Baumgarten CM. Osmotic gradient-induced water permeation across the sarcolemma of rabbit ventricular myocytes. J Gen Physiol. 1996. 107:503–514.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fibroblast-derived interleukin-6 exacerbates adverse cardiac remodeling after myocardial infarction
- KLF9 deficiency protects the heart from inflammatory injury triggered by myocardial infarction
- Evaluation of doppler echocardiographic patterns of left ventricular filling in the patients with recent acute myocardial infarction
- Peiminine inhibits myocardial injury and fibrosis after myocardial infarction in rats by regulating mitogen-activated protein kinase pathway
- Aconitine Intoxication Misdiagnosed as Acute Myocardial Infarction