J Korean Med Sci.
2004 Jun;19(3):333-340. 10.3346/jkms.2004.19.3.333.
Bacterial Growth in Amniotic Fluid Is Dependent on the Iron-Availability and the Activity of Bacterial Iron-Uptake System
- Affiliations
-
- 1Department of Pediatrics, Seonam University Medical School, Namwon, Korea.
- 2Department of Pediatrics, Chosun University Medical School, Gwangju, Korea.
- 3Department of Anatomy, Chosun University Medical School, Gwangju, Korea.
- 4Department of Microbiology and Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea. shsin@chosun.ac.kr
- KMID: 1786808
- DOI: http://doi.org/10.3346/jkms.2004.19.3.333
Abstract
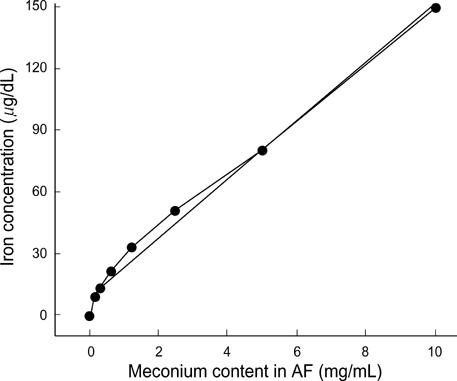
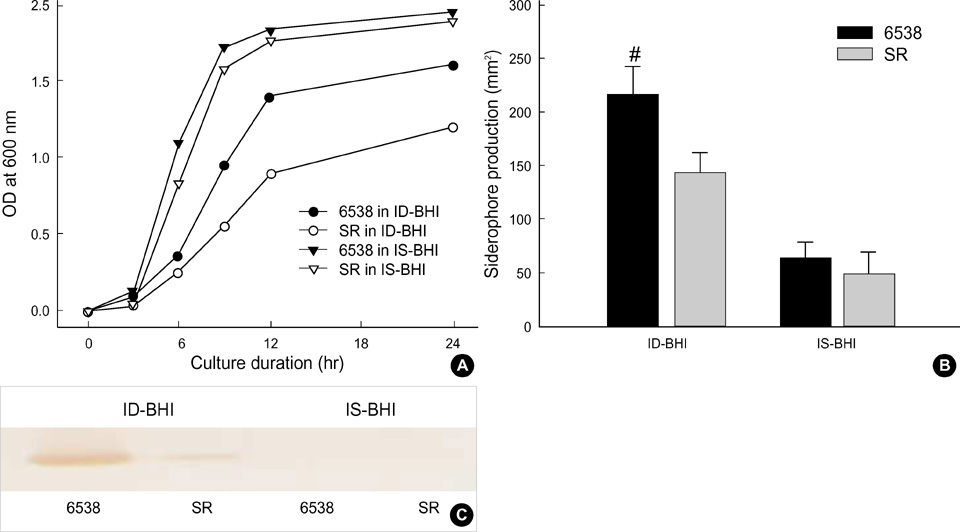
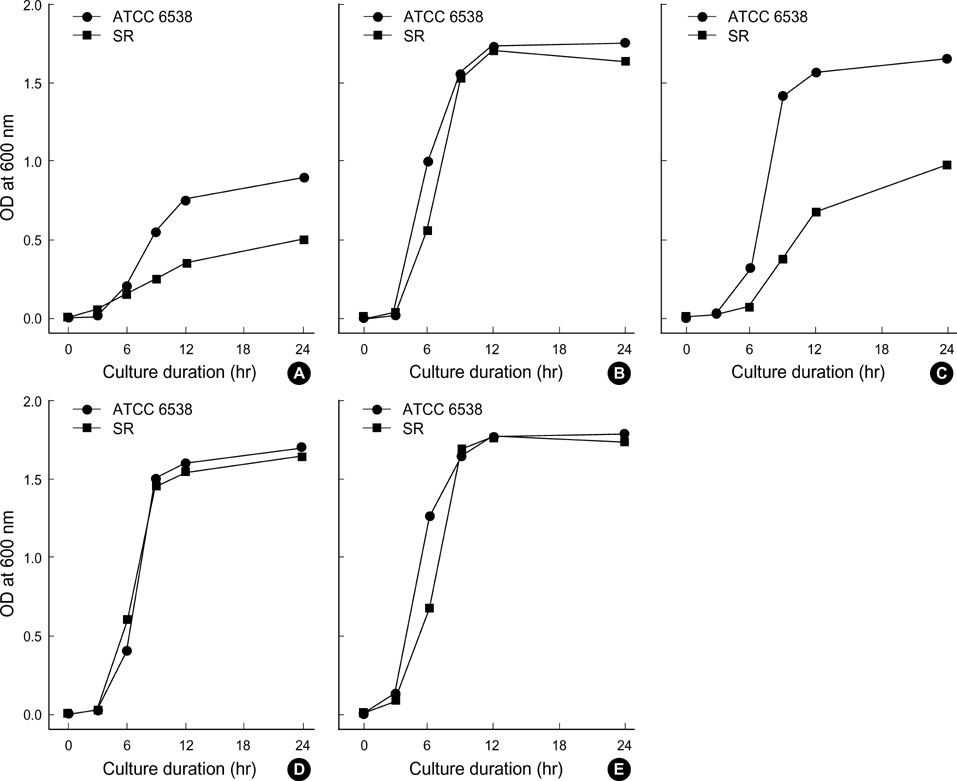
- In the present study, the relationship among iron-availability, antibacterial activity, role of meconium as an iron source and the activity of bacterial iron-uptake system (IUS) for bacterial growth in amniotic fluid (AF) were investigated. Staphylococcus aureus ATCC 6538 and its streptonigrin-resistant (SR) mutant with defective IUS were used as the test strains. The growth of S. aureus in AF was stimulated dosedependently by addition of meconium. Bacterial growth stimulated by meconium was re-inhibited dose-dependently by addition of iron-chelator, dipyridyl and apotransferrin. Iron concentration was correlated with the meconium content in AF (r(2)= 0.989, p=0.001). High-affinity IUS of S. aureus was expressed only in AF but not in AF with meconium. The growth of SR strain was more retarded than that of the parental strain in the iron-deficient brain heart infusion (ID-BHI), clear AF and AF containing apotransferrin. The retarded growth of both strains in the ID-BHI and AF was recovered by addition of holotransferrin, hemoglobin and FeCl3. Taken together, the antibacterial activity of AF is closely related with low iron-availability. Bacterial growth in AF considerably depends on the activity of bacterial IUS. Meconium acts as one of the exogenous iron-sources and thus can stimulate bacterial growth in AF.
MeSH Terms
-
Amniotic Fluid/*microbiology
Antibiotics, Antineoplastic/pharmacology
Chelating Agents/pharmacology
Dose-Response Relationship, Drug
Female
Ferric Compounds/pharmacology
Human
Iron/*metabolism
Ligands
Meconium/metabolism
Mutation
Pregnancy
Pregnancy Trimester, Third
Protein Binding
Staphylococcus aureus/metabolism
Streptonigrin/pharmacology
Support, Non-U.S. Gov't
Time Factors
Figure
Reference
-
1. Abbasi IA, Hemming VG, Eglinton GS, Johnson TR. Proliferation of group B streptococci in human amniotic fluid in vitro. Am J Obstet Gynecol. 1987. 156:95–99.
Article2. Evans HE, Levy E, Glass L. Effect of amniotic fluid on bacterial growth. Obstet Gynecol. 1977. 49:35–37.3. Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970. 106:59–65.
Article4. Honkonen E, Näntö V, Hyörä H, Vuorinen K, Erkkola R. Trace elements and antibacterial activity in amniotic fluid. Biol Neonate. 1986. 50:21–26.
Article5. Oka K, Hagio Y, Tetsuoh M, Kawano K, Hamada T, Kato T. The effect of transferrin and lysozyme on the antibacterial activity of amniotic fluid. Biol Res Pregnancy Perinatol. 1987. 8:1–6.6. Schlievert P, Johnson W, Galask RP. Bacterial growth inhibition by amniotic fluid. VI. Evidence for a zinc-peptide antibacterial system. Am J Obstet Gynecol. 1976. 125:906–910.7. Tran SH, Caughey AB, Musci TJ. Meconium-stained amniotic fluid is associated with puerperal infections. Am J Obstet Gynecol. 2003. 189:746–750.
Article8. Edwards RK. Meconium-stained amniotic fluid and its association with obstetric infections. Prim Care Update OB/Gyns. 1998. 5:315–318.
Article9. Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, Avila C, Nores J, Jimenez C. Meconium-stained amniotic fluid: A risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991. 164:859–862.
Article10. Hoskins IA, Hemming VG, Johnson TR, Winkel CA. Effects of alterations of zinc-to-phosphorus ratios and meconium content on Group B Streptococcus growth in human amniotic fluid in vitro. Am J Obstet Gynecol. 1987. 157:770–773.
Article11. Clark P, Duff P. Inhibition of neutrophil oxidative burst and phagocytosis by meconium. Am J Obstet Gynecol. 1995. 173:1301–1305.
Article12. Florman AL, Teubner D. Enhancement of bacterial growth in amniotic fluid by meconium. Brief clinical and laboratory observations. J Pediatr. 1969. 74:111–114.13. Friel JK, Matthew JD, Andrews WL, Skinner CT. Trace elements in meconium from preterm and full-term infants. Biol Neonate. 1989. 55:214–217.
Article14. Larsen B, Snyder IS, Galask RP. Transferrin concentration in human amniotic fluid. Am J Obstet Gynecol. 1973. 117:952–954.
Article15. Auger P, Marquis G, Dallaire L, Joly J. Stunted growth Candida albicans in human amniotic fluid in vitro. J Lab Clin Med. 1980. 95:272–281.16. Thadepalli H, Gangopadhyay PK, Maidman JE. Amniotic fluid analysis for antimicrobial factors. Int J Gynaecol Obstet. 1982. 20:65–72.
Article17. Trivier D, Courcol RJ. Iron depletion and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 1996. 141:117–127.18. Lim Y, Shin SH, Lee SI, Kim IS, Rhee JH. Iron repressibility of siderophore and transferrin-binding protein in Staphylococcus aureus. FEMS Microbiol Lett. 1998. 163:19–24.19. Taylor JM, Heinrichs DE. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol Microbiol. 2002. 43:1603–1614.
Article20. Chung JH, Park MH, Kim JH, Lim Y, Shin SH. Growth and siderophore production of staphylococci in human peritoneal dialysate. J Korean Med Sci. 2003. 18:158–162.
Article21. Leong SA, Neilands JB. Siderophore production by phytopathogenic microbial species. Arch Biochem Biophys. 1982. 218:351–359.
Article22. Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982. 22:961–968.
Article23. Shin SH, Lim Y, Lee SE, Yang NW, Rhee JH. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J Microbiol Methods. 2001. 44:89–95.
Article24. Cheung AL, Fischetti VA. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988. 56:1061–1065.25. Weinberg ED, Weinberg GA. The role of iron in infection. Curr Opin Infect Dis. 1995. 8:164–169.
Article26. Neilands JB. Siderophores: structure and function of microbial iron compounds. J Biol Chem. 1995. 270:26723–26726.27. Simpson LM, Oliver JD. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding proteins. Curr Microbiol. 1987. 15:155–157.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Iron-Uptake System on the Growth of Staphylococcus aureus according to the Iron Concentration and Oxygen Tension
- Multiple High-affinity Iron-uptake Systems of Vibrio vulnificus
- Meconium as an Iron Source for the Growth of Staphylococcus Aureus in Amniotic Fluid
- Characterization of the hemin-binding property of Porphyromonas endodontalis
- Effect of Iron-Uptake systems on the Growth of Staphylococcus aureus in Amniotic Fluid