J Korean Med Sci.
2004 Feb;19(1):113-122. 10.3346/jkms.2004.19.1.113.
Continuous Brain-derived Neurotrophic Factor (BDNF) Infusion After Methylprednisolone Treatment in Severe Spinal Cord Injury
- Affiliations
-
- 1Department of Neurosurgery, Stanford University School of Medicine.
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea. spine@snuh.org
- KMID: 1785704
- DOI: http://doi.org/10.3346/jkms.2004.19.1.113
Abstract
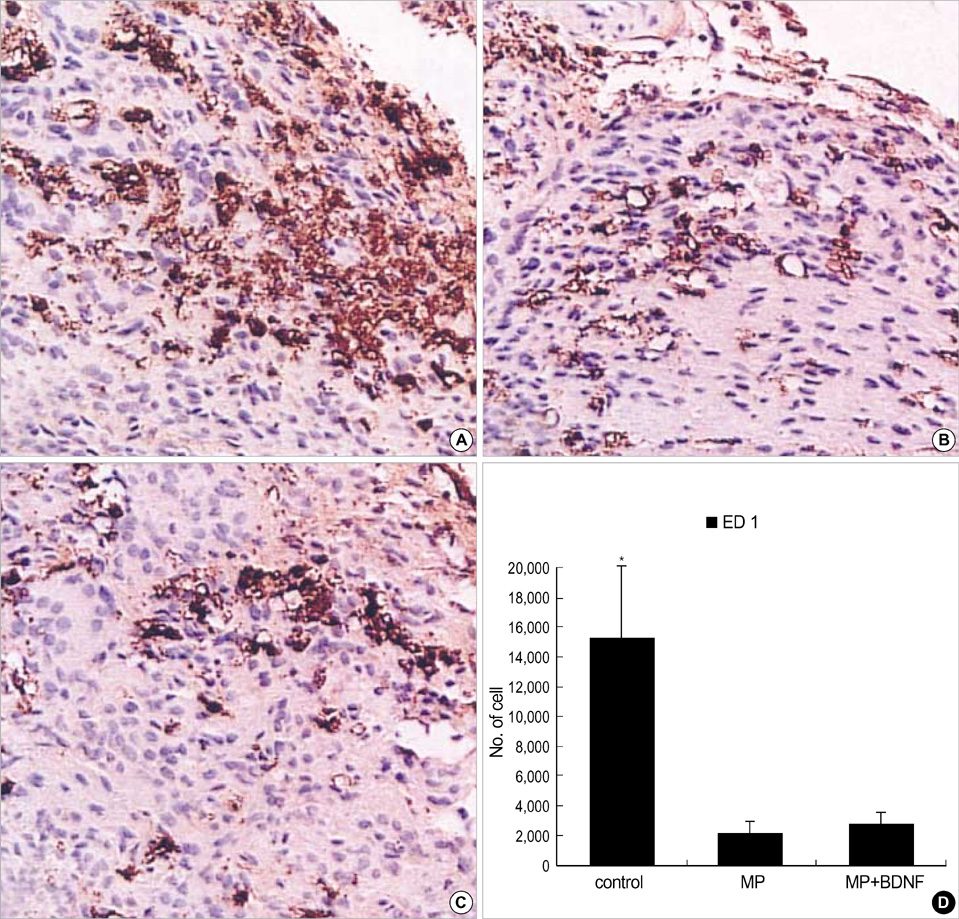
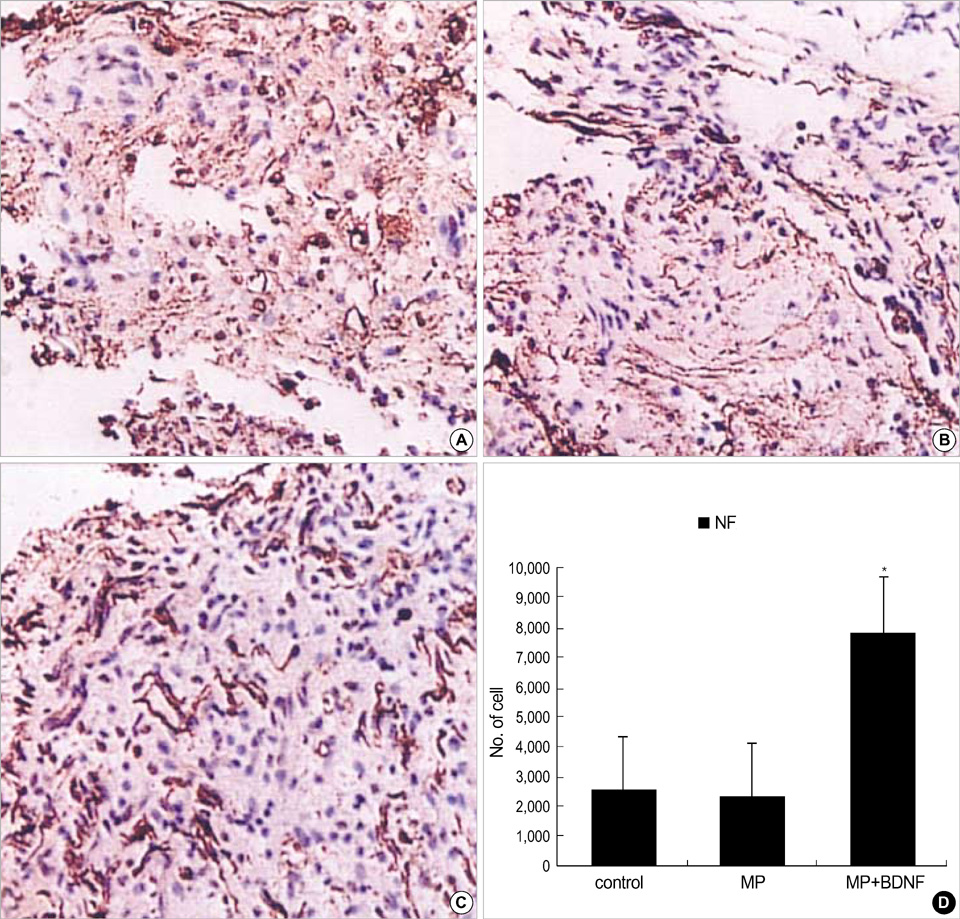
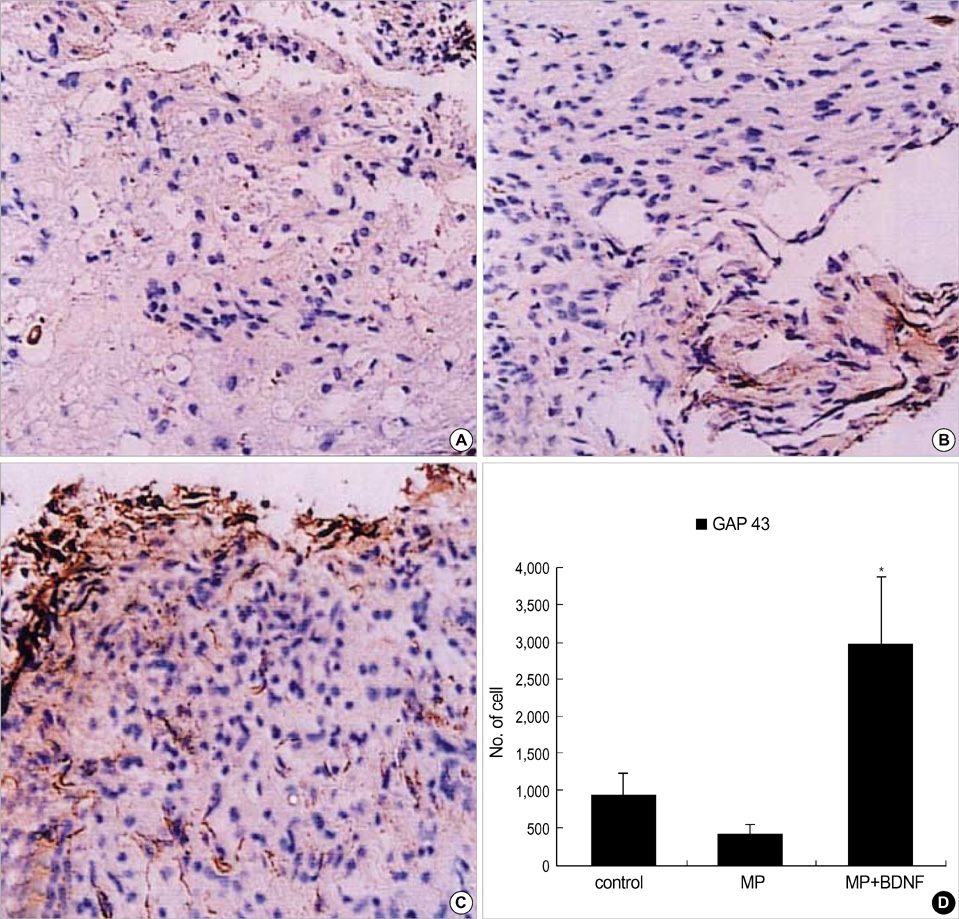
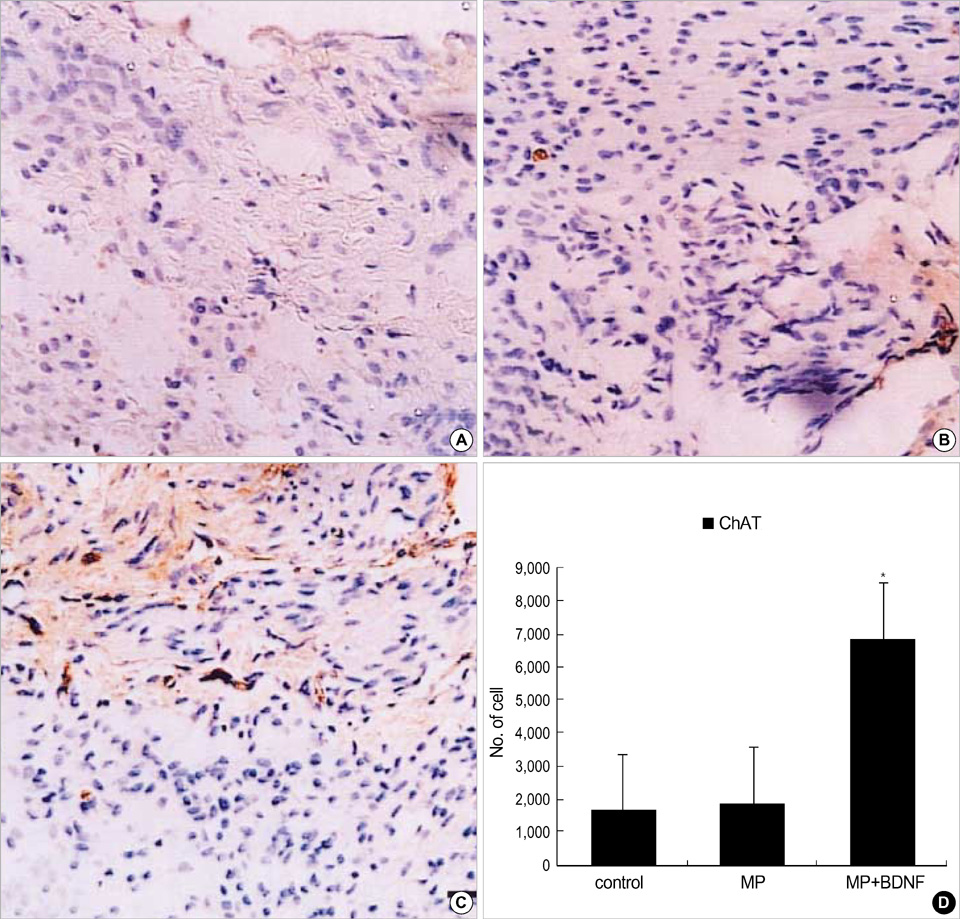
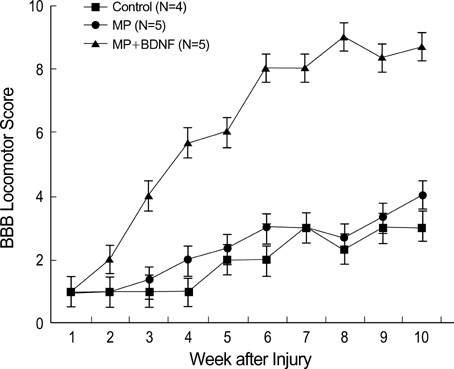
- Although methylprednisolone (MP) is the standard of care in acute spinal cord injury (SCI), its functional outcome varies in clinical situation. Recent report demonstrated that MP depresses the expression of growth-promoting neurotrophic factors after acute SCI. The present study was designed to investigate whether continuous infusion of brain-derived neurotrophic factor (BDNF) after MP treatment promotes functional recovery in severe SCI. Contusion injury was produced at the T10 vertebral level of the spinal cord in adult rats. The rats received MP intravenously immediately after the injury and BDNF was infused intrathecally using an osmotic mini-pump for six weeks. Immunohistochemical methods were used to detect ED-1, Growth associated protein-43 (GAP-43), neurofilament (NF), and choline acethyl transferase (ChAT) levels. BDNF did not alter the effect of MP on hematogenous inflammatory cellular infiltration. MP treatment with BDNF infusion resulted in greater axonal survival and regeneration compared to MP treatment alone, as indicated by increases in NF and GAP-43 gene expression. Adjunctive BDNF infusion resulted in better locomotor test scores using the Basso-Beattie-Bresnahan (BBB) test. This study demonstrated that continuous infusion of BDNF after initial MP treatment improved functional recovery after severe spinal cord injury without dampening the acute effect of MP.
MeSH Terms
-
Animals
Anti-Inflammatory Agents/pharmacology
Axons/pathology
Brain-Derived Neurotrophic Factor/metabolism/*pharmacology
Choline O-Acetyltransferase/metabolism
Female
GAP-43 Protein/metabolism
Gene Expression Regulation
Immunohistochemistry
Methylprednisolone/metabolism/*pharmacology
Osmosis
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Spinal Cord/pathology
Spinal Cord Injuries/*pathology
Time Factors
Figure
Cited by 1 articles
-
Effects of Methylprednisolone on the Neural Conduction of the Motor Evoked Potentials in Spinal Cord Injured Rats
Bae Hwan Lee, Kyung Hee Lee, Do Heum Yoon, Un Jeng Kim, Yong Soon Hwang, Sang Keun Park, Joong Uhn Choi, Yong Gou Park
J Korean Med Sci. 2005;20(1):132-138. doi: 10.3346/jkms.2005.20.1.132.
Reference
-
1. Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999. 44:1027–1039.
Article2. Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, Gropper MR, Goffin J, Madsen PW 3rd, Maiman DJ, Ondra SL, Rosner M, Sasso RC, Trost GR, Zeidman S. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000. 13:185–199.
Article3. Hadley MN, Walters BC, Grabb PA, Oyesiku NM, Przybylski GJ, Resnick DK, Ryken TC, Mielke DH. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002. 49:407–498.4. Bracken MB. Pharmacological treatment of acute spinal cord injury: current status and future projects. J Emerg Med. 1993. 11:Suppl 1. 43–48.5. Galandiuk S, Raque G, Appel S, Polk HC Jr. The two-edged sword of large-dose steroids for spinal cord trauma. Ann Surg. 1993. 218:419–425.
Article6. George ER, Scholten DJ, Buechler CM, Jordan-Tibbs J, Mattice C, Albrecht RM. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am Surg. 1995. 61:659–663.7. Gerndt SJ, Rodriguez JL, Pawlik JW, Taheri PA, Wahl WL, Micheals AJ, Papadopoulos SM. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. 1997. 42:279–284.
Article8. Gerhart KA, Johnson RL, Menconi J, Hoffman RE, Lammertse DP. Utilization and effectiveness of methylprednisolone in a population-based sample of spinal cord injured persons. Paraplegia. 1995. 33:316–321.
Article9. Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000. 17:203–218.
Article10. Lindsay RM, Thoenen H, Barde YA. Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev Biol. 1985. 112:319–328.
Article11. Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990. 5:297–306.
Article12. Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998. 18:2808–2821.
Article13. Murphy GM Jr, Jia XC, Yu AC, Lee YL, Tinklenberg JR, Eng LF. Reverse transcription and polymerase chain reaction technique for quantification of mRNA in primary astrocyte cultures. J Neurosci Res. 1993. 35:643–651.
Article14. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.
Article15. Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985. 54:589–599.16. Xu J, Qu ZX, Hogan EL, Perot PL Jr. Protective effect of methylprednisolone on vascular injury in rat spinal cord injury. J Neurotrauma. 1992. 9:245–253.
Article17. Bracken MB, Shepard MJ, Collins WF Jr, Holford TR, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon JC, Marshall LF. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992. 76:23–31.18. Brook GA, Plate D, Franzen R, Martin D, Moonen G, Schoenen J, Schmitt AB, Noth J, Nacimiento W. Spontaneous longitudinally orientated axonal regeneration is associated with the Schwann cell framework within the lesion site following spinal cord compression injury of the rat. J Neurosci Res. 1998. 53:51–65.
Article19. Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp Neurol. 1994. 126:1–14.
Article20. Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and alpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997. 17:9583–9595.21. Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998. 18:3803–3815.22. Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997. 144:273–286.
Article23. Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000. 7:574–585.
Article24. Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985. 8:233–261.
Article25. Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998. 154:170–184.
Article26. Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991. 65:885–893.
Article27. Seeburger JL, Tarras S, Natter H, Springer JE. Spinal cord motoneurons express p75NGFR and p145trkB mRNA in amyotrophic lateral sclerosis. Brain Res. 1993. 621:111–115.
Article28. Frisen J, Verge VM, Cullheim S, Persson H, Fried K, Middlemas DS, Hunter T, Hokfelt T, Risling M. Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc Natl Acad Sci USA. 1992. 89:11282–11286.
Article29. Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993. 10:359–367.
Article30. DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992. 8:983–993.
Article31. Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993. 24:1555–1577.32. Yan Q, Matheson C, Lopez OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J Neurosci. 1994. 14:5281–5291.33. Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, Zlotchenko E, Lindsay RM, Liu L. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995. 15:1044–1056.
Article34. Novikov L, Novikova L, Kellerth JO. Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci Lett. 1995. 200:45–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of BDNF (Brain Derived Neurotrophic Factor) Expression Associated with Urodynamic Changes in Rat Spinal Cord Injury Animal Model
- Expression and Distribution of BDNF (Brain Derived Neurotrophic Factor) in the Rat Hypothalamus
- The effect of 4-Methylcatechol treatment in chronic constrictive injury of the rat sciatic nerve on the allodynia and spinal neurotrophic factors
- Effects of Peripheral Inflammation on Brain-Derived Neurotrophic Factor and TrkB in Rat Dorsal Root Ganglia and Spinal Cord
- Epigenetic Regulation in the Brain after Spinal Cord Injury : A Comparative Study