J Vet Sci.
2014 Jun;15(2):259-265. 10.4142/jvs.2014.15.2.259.
Extent of Mycobacterium bovis infection in dairy cattle herds subject to partial culling as determined by an interferon-gamma assay
- Affiliations
-
- 1Department of Microbiology and Institute for Immunology and Immunological Diseases, Brain Korea 21 Plus Project for the Medical Sciences, Yonsei University College of Medicine, Seoul 120-752, Korea. raycho@yuhs.ac
- 2Gyeonggido Veterinary Service, Yangju 482-020, Korea.
- KMID: 1784647
- DOI: http://doi.org/10.4142/jvs.2014.15.2.259
Abstract
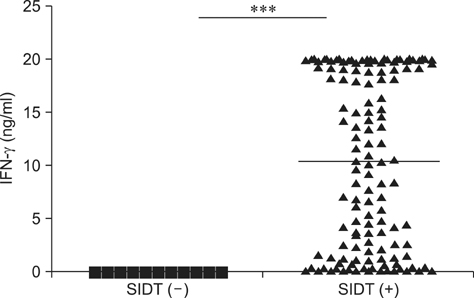
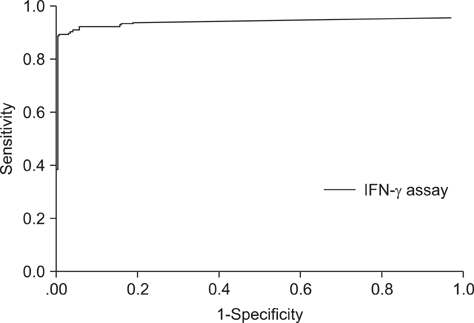
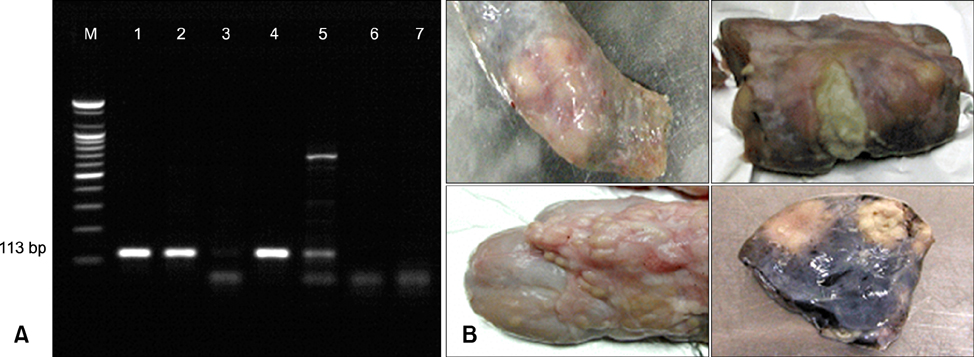
- The interferon-gamma (IFN-gamma) assay is employed as a complementary diagnostic test for bovine tuberculosis (BTB) in many countries. To simplify this assay, we established a 96-well plate format using the ESAT-6 and CFP-10 antigens and then employed it to determine the extent of Mycobacterium (M.) bovis infection in dairy herds with a history of BTB outbreaks in a country where only selective culling is practiced. The sensitivity and specificity of this IFN-gamma assay were 85.9% and 100%, respectively, based on comparison with the conventional single intradermal tuberculin test (SIDT). The IFN-gamma assay was also positive in 30.4% and 36.8% of SIDT-negative animals from herds with recent and remote BTB outbreaks, respectively. Of 14 SIDT-negative, IFN-gamma positive cattle, five (35.7%) were culture positive and an additional six were positive based on a polymerase chain reaction-based test for M. bovis. Therefore, the IFN-gamma assay has the potential to serve as a specific and sensitive test for M. bovis infection in dairy cattle. Further, the results indicated that a substantial portion of SIDT-negative animals in herds with previous BTB outbreaks were actually infected with M. bovis. Accordingly, the present selective-culling strategy may require modifications to include this more sensitive assay.
MeSH Terms
-
Animals
Antigens, Bacterial/*diagnostic use
Bacterial Proteins/diagnostic use
Cattle
Female
Interferon-gamma Release Tests/*veterinary
Mycobacterium bovis/*isolation & purification
Polymerase Chain Reaction/veterinary
Republic of Korea/epidemiology
Tuberculosis, Bovine/*diagnosis/*epidemiology/microbiology
Antigens, Bacterial
Bacterial Proteins
Figure
Reference
-
1. Aagaard C, Govaerts M, Meikle V, Vallecillo A, Gutierrez-Pabello JA, Suarez-Güemes F, McNair J, Cataldi A, Espitia C, Andersen P, Pollock JM. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J Clin Microbiol. 2006; 44:4326–4335.
Article2. Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW. Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Vet Microbiol. 2001; 80:37–46.
Article3. de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006; 81:190–210.
Article4. Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, Vedvick T, Badaro R, Reed SG, Houghton R. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000; 38:3285–3290.
Article5. González Llamazares OR, Gutiérrez Martín CB, Alvarez Nistal D, de la Puente Redondo VA, Dominguez Rodríguez L, Rodríquez Ferri E. Field evaluation of the single intradermal cervical tuberculin test and the interferon-γ assay for detection and eradication of bovine tuberculosis in Spain. Vet Microbiol. 1999; 70:55–66.
Article6. Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin Exp Immunol. 2005; 141:432–439.
Article7. Lilenbaum W, Schettini JC, Souza GN, Ribeiro ER, Moreira EC, Fonseca LS. Comparison between a γ-IFN assay and intradermal tuberculin test for the diagnosis of bovine tuberculosis in field trials in Brazil. Zentralbl Veterinarmed B. 1999; 46:353–358.
Article8. Mathews F, Macdonald DW, Taylor GM, Gelling M, Norman RA, Honess PE, Foster R, Gower CM, Varley S, Harris A, Palmer S, Hewinson G, Webster JP. Bovine tuberculosis (Mycobacterium bovis) in British farmland wildlife: the importance to agriculture. Proc Biol Sci. 2006; 273:357–365.
Article9. Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ. The tuberculin test. Vet Microbiol. 1994; 40:111–124.
Article10. Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Vet Microbiol. 1994; 40:153–177.11. Mustafa AS, Cockle PJ, Shaban F, Hewinson RG, Vordermeier HM. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin Exp Immunol. 2002; 130:37–42.
Article12. Neill SD, Cassidy J, Hanna J, Mackie DP, Pollock JM, Clements A, Walton E, Bryson DG. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet Rec. 1994; 135:134–135.
Article13. Pollock JM, Girvin RM, Lightbody KA, Clements RA, Neill SD, Buddle BM, Andersen P. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet Rec. 2000; 146:659–665.
Article14. Pollock JM, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect Immun. 1997; 65:2587–2592.
Article15. Rhodes SG, Palmer N, Graham SP, Bianco AE, Hewinson RG, Vordermeier HM. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect Immun. 2000; 68:5393–5400.
Article16. Rothel JS, Jones SL, Corner LA, Cox JC, Wood PR. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust Vet J. 1992; 69:1–4.
Article17. Scacchia M, Lelli R, Petrini A, Prencipe V, Calistri P, Giovannini A. Use of innovative methods in the eradication of bovine tuberculosis. J Vet Med B Infect Dis Vet Public Health. 2000; 47:321–327.
Article18. Taylor GM, Worth DR, Palmer S, Jahans K, Hewinson RG. Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesions using PCR. BMC Vet Res. 2007; 3:12.
Article19. Wang BL, Xu Y, Wu CQ, Xu YM, Wang HH. Cloning, expression, and refolding of a secretory protein ESAT-6 of Mycobacterium tuberculosis. Protein Expr Purif. 2005; 39:184–188.
Article20. Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, Cousins DB, McCormick BS, Francis BR, Creeper J, Tweddle NE. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. 1991; 68:286–290.
Article21. Wood PR, Corner LA, Rothel JS, Ripper JL, Fifis T, McCormick BS, Francis B, Melville L, Small K, de Witte K, Tolson J, Ryan TJ, de Lisle GW, Cox JC, Jones SL. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992; 31:71–79.
Article22. Wood PR, Jones SL. BOVIGAM™: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinb). 2001; 81:147–155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advancing parity is associated with high milk production at the cost of body condition and increased periparturient disorders in dairy herds
- Serum interferon-gamma and interleukins-6 and -8 during infection with Fasciola gigantica in cattle and buffaloes
- Comparison of tuberculin skin test with Interferon-gamma assay for the diagnosis of bovine tuberculosis in Korean cattle
- Molecular fingerprinting of clinical isolates of Mycobacterium bovis and Mycobacterium tuberculosis from India by restriction fragment length polymorphism (RFLP)
- Diagnosis of Mycobacterium tuberculosis Infection using Ex-vivo interferon-gamma Assay