Tuberc Respir Dis.
2006 May;60(5):497-509.
Diagnosis of Mycobacterium tuberculosis Infection using Ex-vivo interferon-gamma Assay
- Affiliations
-
- 1Division of Pulmonary Medicine, Department of Internal Medicine, Konkuk University Medical Center, Chungju Hospital, Korea.
- 2Division of Pulmonary and Critical Care Medicine, University of Ulsan College of Medicine, Asan Medical Center, Korea. shimts@amc.seoul.kr
Abstract
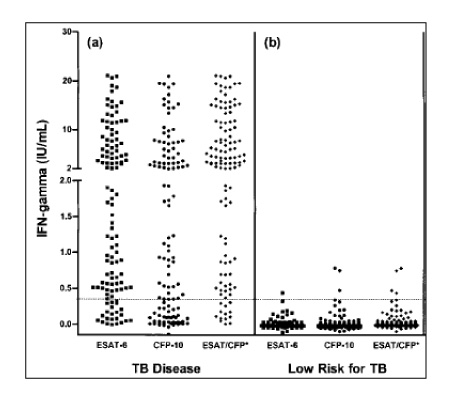
- Until recently, the tuberculin skin test (TST) has been the only tool available for diagnosing a latent TB infection. However, the development of new diagnostic tools, using the Mycobacterium tuberculosis (MTB)-specific early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) antigens, should improve the control of tuberculosis (TB) by allowing a more accurate identification of a latent TB infection (LTBI). Antigen-specific interferon-gamma (IFN-gamma) assays have greater specificity in BCG-vaccinated individuals, and as less biased by nontuberculous mycobacterial infections. Many comparative studies have suggested that those assays have a higher specificity than the TST, and the sensitivity of these assays are expected to remarkably improved if more MTB-specific antigens can become available. Nevertheless, the major obstacle to the widespread use of these tests is the limited financial resources. Similar to other diagnostic tests, the predictive value of IFN-gamma assays depends on the prevalence of a MTB infection in the population being tested. Therefore, prospective studies will be meeded to establish the applicability of these new assays at multiple geographic locations among patients of different ethnicities, and to determine if the IFN-gamma responses can indicate those with a high risk of progressing to active TB.
MeSH Terms
Figure
Reference
-
1. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993. 178:2243–2247.2. Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996. 335:1941–1949.3. Wongtim S, Silachamroon U, Ruxrungtham K, Udompanich V, Limthongkul S, Charoenlap P, et al. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax. 1999. 54:921–924.4. Ogawa K, Koga H, Yang B, Fukuda M, Ohno H, Yamamoto Y, et al. Differential diagnosis of tuberculous pleurisy by the measurement of cytokine concentration in pleural effusion. Kekkaku. 1996. 71:663–669.5. Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990. 145:149–154.6. Ribera E, Martinez Vasquez JM, Ocana I, Ruiz I, Jiminez JG, Encabo G, et al. Diagnostic value of ascites gamma interferon levels in tuberculous peritonitis: comparison with adenosine deaminase activity. Tubercle. 1991. 72:193–197.7. Burgess LJ, Reuter H, Carstens ME, Taljaard JJ, Doubell AF. The use of adenosine deaminase and interferon-gamma as diagnostic tools for tuberculous pericarditis. Chest. 2002. 122:900–905.8. Ribeiro-Rodrigues R, Resende Co T, Johnson JL, Ribeiro F, Palaci M, Sa RT, et al. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002. 9:818–823.9. Tsao TC, Huang CC, Chiou WK, Yang PY, Hsieh MJ, Tsao KC. Levels of interferon-gamma and interleukin-2 receptor-alpha for bronchoalveolar lavage fluid and serum were correlated with clinical grade and treatment of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002. 6:720–727.10. Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, et al. Six-month therapy with aerosolized interferon-gamma for refractory multidrug-resistant pulmonary tuberculosis. J Korean Med Sci. 2004. 19:167–171.11. Suarez-Mendez R, Garcia-Garcia I, Fernandez-Olivera N, Valdes-Quintana M, Milanes-Virelles MT, Carbonell D, et al. Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2004. 4:44.12. Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997. 349:1513–1515.13. Kim EK, Shim TS, Lee JY, Oh YM, Lim CM, Lee SD, et al. The adjuvant effect of subcutaneous interferon-gamma in the treatment of refractory multidrug-resistant pulmonary tuberculosis. Tuberc Respir Dis. 2004. 57:226–233.14. Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001. 286:1740–1747.15. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993. 17:968–975.16. Jasmer RM, Nahid P, Hopewell PC. Clinical practice: latent tuberculosis infection. N Engl J Med. 2002. 347:1860–1866.17. Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004. 170:59–64.18. Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004. 170:65–69.19. Mazurek GH, Jereb J, LoBue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005. 54:49–55.20. Cellestis Ltd. QuantiFERON-TB Gold (In-Tube Method) package insert. Cat. no. 0599 0201. 2004. Victoria, Australia:21. Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002. 16:2285–2293.22. Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in the Gambia. Clin Infect Dis. 2004. 38:966–973.23. Arend SM, Engelhard AC, Groot G, de Boer K, Andersen P, Ottenhoff TH, et al. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin Diagn Lab Immunol. 2001. 8:1089–1096.24. Lein AD, von Reyn CF, Ravn P, Horsburgh CR Jr, Alexander LN, Andersen P. Cellular immune responses to ESAT-6 discriminate between patients with pulmonary disease due to Mycobacterium avium complex and those with pulmonary disease due to Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 1999. 6:606–609.25. Wood PR, Corner LA, Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990. 49:46–49.26. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998. 5:531–536.27. Mazurek GH, Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep. 2003. 52:15–18.28. Fietta A, Meloni F, Cascina A, Morosini M, Marena C, Troupioti P, et al. Comparison of a whole-blood interferon-gamma assay and tuberculin skin testing in patients with active tuberculosis and individuals at high or low risk of Mycobacterium tuberculosis infection. Am J Infect Control. 2003. 31:347–353.29. Johnson PD, Stuart RL, Grayson ML, Olden D, Clancy A, Ravn P, et al. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin Diagn Lab Immunol. 1999. 6:934–937.30. Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis-infection in healthy urban Indians. J Infect Dis. 2001. 183:469–477.31. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005. 293:2756–2761.32. Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a commercial whole blood interferon-gamma assay for tuberculosis infection. Am J Respir Crit Care Med. 2005. 172:631–635.33. Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. Tuberculosis specific interferon gamma test. Respir Res. 2006. 7:56.34. Mahomed H, Hughes EJ, Hawkridge T, Minnies D, Simon E, Little F, et al. Comparison of mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006. 10:310–316.35. Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001. 163:824–828.36. Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001. 167:5217–5225.37. Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998. 95:270–275.38. Pathan AA, Wilkinson KA, Wilkinson RJ, Latif M, McShane H, Pasvol G, et al. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000. 30:2713–2721.39. Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001. 357:2017–2021.40. Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003. 361:1168–1173.41. Shams H, Weis SE, Klucar P, Lalvani A, Moonan PK, Pogoda JM, et al. Enzyme-linked immunospot and tuberculin skin testing to detect latent tuberculosis infection. Am J Respir Crit Care Med. 2005. 172:1161–1168.42. Richeldi L, Ewer K, Losi M, Bergamini BM, Roversi P, Deeks J, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004. 170:288–295.43. American Thoracic Society/Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000. 161:S221–S247.44. Piana F, Codecasa LR, Besozzi G, Migliori GB, Cirillo DM. Use of commercial interferon-gamma assays in immunocompromised patients for tuberculosis diagnosis. Am J Respir Crit Care Med. 2006. 173:130.45. Piana F, Codecasa LR, Cavallerio P, Ferrarese M, Migliori GB, Barbarano L, et al. Use of a T-cell based test for detection of TB infection among immunocompromised patients. Eur Respir J. 2006. (In press).46. Scholvinck E, Wilkinson KA, Whelan AO, Martineau AR, Levin M, Wilkinson RJ. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and enzyme-linked immunospot methods. J Clin Microbiol. 2004. 42:829–831.47. Goletti D, Vincenti D, Carrara S, Butera O, Bizzoni F, Bernardini G, et al. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin Diagn Lab Immunol. 2005. 12:1311–1316.48. Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006. (In press).49. Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006. 367:1328–1334.50. Stuart RL, Olden D, Johnson PD, Forbes A, Bradley PM, Rothel JS, et al. Effect of anti-tuberculosis treatment on the tuberculin interferon-gamma response in tuberculin skin test (TST) positive health care workers and patients with tuberculosis. Int J Tuberc Lung Dis. 2000. 4:555–561.51. Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004. 38:754–756.52. Markova R, Drenska R, Terzieva V, Todorova Y, Dimitrov V, Nikolova M. The application of the T spot-TB assay in Bulgarian patients with tuberculosis infection. Probl Infect Parasit Dis. 2005. 33:10–11.53. Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah DJ, Lugos MD, et al. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006. 6:66.54. Kim YS, Cho SA, Park MS, Chung JH, Hwang SY, Kim SK, et al. Change of Interferon gamma production in patients with active tuberculosis after treatment. Am J Respir Crit Care Med. 2004. A376.55. Zinkernagel RM, Bachmann MF, Kundig TM, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996. 14:333–367.56. Hengel RL, Allende MC, Dewar RL, Metcalf JA, Mican JM, Lane HC. Increasing CD4+ T cells specific for tuberculosis correlate with improved clinical immunity after highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2002. 18:969–975.57. Wu-Hsieh BA, Chen CK, Chang JH, Lai SY, Wu CH, Cheng WC, et al. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin Infect Dis. 2001. 33:1336–1340.58. Hill PC, Fox A, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, et al. Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin Infect Dis. 2005. 40:273–278.59. Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol. 2002. 169:2210–2214.60. Sousa AO, Wargnier A, Poinsignon Y, Simonney N, Gerber F, Lavergne F, et al. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tuber Lung Dis. 2000. 80:27–33.61. Park IN. Evaluation of the response to antituberculous treatment using ex-vivo interferon-gamma assay. Tuberc Respir Dis. 2005. 59:55.62. Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosisof tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004. 364:2196–2203.63. Ravn P, Munk ME, Andersen AB, Lundgren B, Lundgren JD, Nielsen LN, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005. 12:491–496.64. Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin Diagn Lab Immunol. 2001. 8:571–578.65. Liu XQ, Dosanjh D, Varia H, Ewer K, Cockle P, Pasvol G, et al. Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection. Infect Immun. 2004. 72:2574–2581.66. Brock I, Weldingh K, Leyten EM, Arend SM, Ravn P, Andersen P. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J Clin Microbiol. 2004. 42:2379–2387.67. Wrighton-Smith P, Zellweger JP. Direct costs of three models for the screening of latent tuberculosis infection. Eur Respir J. 2006. (In press).68. Doherty TM, Demissie A, Olobo J, Wolday D, Britton S, Eguale T, et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol. 2002. 40:704–706.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three Cases of Erythema Induratum Diagnosed by Histopathology and Interferon-Γ Release Assay
- Interferon-gamma Release Assay Using Pericardial Fluid and Peripheral Blood for the Diagnosis of Tuberculous Pericarditis: A Case Report
- Diagnosis and treatment of latent tuberculosis infection
- Effect of Tuberculin Skin Test on Ex-vivo Interferon-gamma Assay for Latent Tuberculosis Infection
- Application of the QuantiFERON(R)-TB Gold Test in Two Cases of Erythema Induratum of Bazin