J Vet Sci.
2014 Jun;15(2):241-248. 10.4142/jvs.2014.15.2.241.
Isolation, in vitro propagation, genetic analysis, and immunogenic characterization of an Ehrlichia canis strain from southeastern Brazil
- Affiliations
-
- 1Institute of Biomedical Sciences, Federal University of Uberlandia, Uberlandia 38405-320, Brazil. rnalves@icbim.ufu.br
- 2Federal Institute of Education, Science and Technology of Triangulo Mineiro, Campus Uberlandia, Uberlandia 38400-974, Brazil.
- 3Institute of Genetics and Biochemistry, Federal University of Uberlandia, Uberlandia 38400-902, Brazil.
- 4Department of Preventive Veterinary Medicine and Animal Health, College of Veterinary Medicine, University of Sao Paulo, Sao Paulo 05508-270, Brazil.
- KMID: 1784645
- DOI: http://doi.org/10.4142/jvs.2014.15.2.241
Abstract
- Amplification of the 16S rRNA gene from a blood sample obtained from a dog in southeastern Brazil was used to confirm a naturally acquired Ehrlichia (E.) canis infection. Following isolation and culturing of the new bacterial strain called Uberlandia, partial sequences of the dsb and p28 genes were obtained. The dsb partial sequence of the novel strain was 100% similar to dsb gene sequences of E. canis obtained from different geographic areas around the world. Conversely, the p28 partial sequence for the E. canis Uberlandia strain differed at several nucleotides from other sequences available in GenBank. To confirm the antigenic profile of the Uberlandia strain, an indirect immunofluorescence assay against E. canis antigens was performed using dog sera collected from two different areas in Brazil (Uberlandia and Sao Paulo). The results suggest that both antigens were able to identify animals seropositive for E. canis in Brazil since these Brazilian strains appear to be highly conserved.
Keyword
MeSH Terms
-
Animals
Antigens, Bacterial/blood/*diagnostic use
Bacterial Outer Membrane Proteins/genetics/metabolism
Bacterial Proteins/*genetics/metabolism
Base Sequence
Brazil
Dog Diseases/diagnosis/*microbiology
Dogs
Ehrlichia canis/*genetics/*immunology/isolation & purification
Ehrlichiosis/diagnosis/microbiology/*veterinary
Fluorescent Antibody Technique, Indirect/veterinary
Male
Molecular Sequence Data
Polymerase Chain Reaction/veterinary
RNA, Ribosomal, 16S/genetics/metabolism
Sequence Alignment/veterinary
Antigens, Bacterial
Bacterial Outer Membrane Proteins
Bacterial Proteins
RNA, Ribosomal, 16S
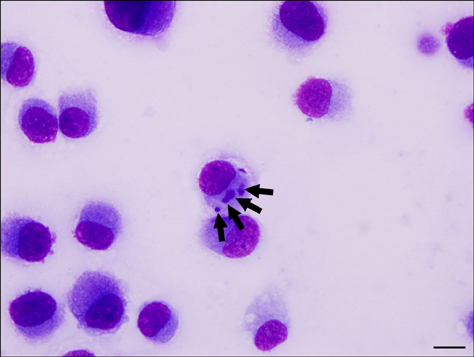
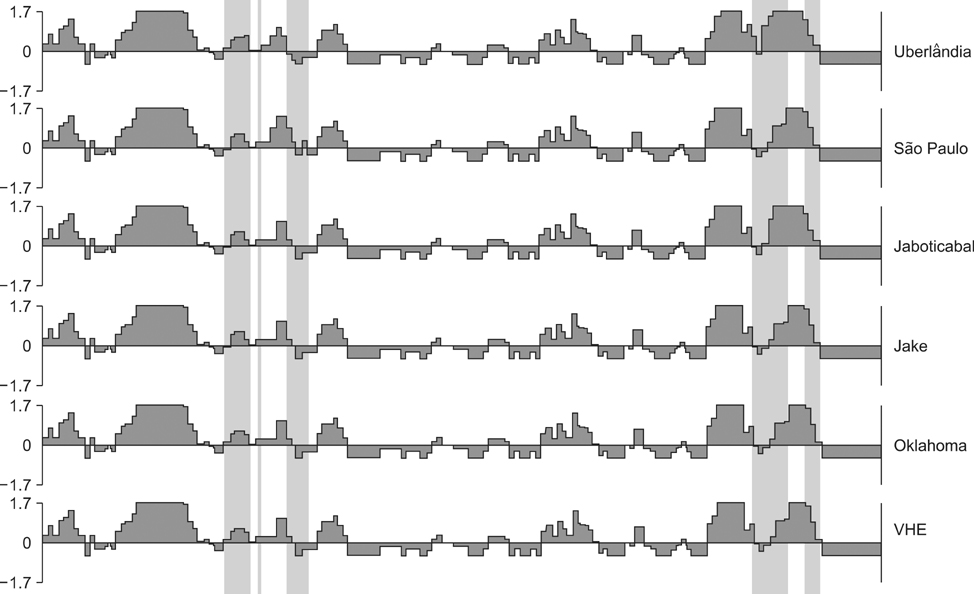
Figure
Reference
-
1. Aguiar DM, Hagiwara MK, Labruna MB. In vitro isolation and molecular characterization of an Ehrlichia canis strain from São Paulo, Brazil. Braz J Microbiol. 2008; 39:489–493.
Article2. Aguiar DM, Saito TB, Hagiwara MK, Machado RZ, Labruna MB. Serological diagnosis of canine monocytic ehrlichiosis with Brazilian antigen of Ehrlichia canis. Cienc Rural. 2007; 37:796–802.3. Aguiar DM, Zhang X, Melo AL, Pacheco TA, Meneses AM, Zanutto MS, Horta MC, Santarém VA, Camargo LM, McBride JW, Labruna MB. Genetic diversity of Ehrlichia canis in Brazil. Vet Microbiol. 2013; 164:315–321.4. Aguirre E, Ayllón T, Sainz A, Amasutegui I, Villaescusa A, Rodríquez-Franco F, Tesouro MA. Results from an indirect fluorescent antibody test using three different strains of Ehrlichia canis. Vet J. 2009; 182:301–305.
Article5. Aguirre E, Sainz A, Dunner S, Amusategui I, López L, Rodríguez-Franco F, Luaces I, Cortés O, Tesouro MA. First isolation and molecular characterization of Ehrlichia canis in Spain. Vet Parasitol. 2004; 125:365–372.
Article6. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215:403–410.
Article7. André MR, Adania CH, Machado RZ, Allegretti SM, Felippe PA, Silva KF, Nakaghi AC. Molecular and serologic detection of Ehrlichia spp. in endangered Brazilian wild captive felids. J Wildl Dis. 2010; 46:1017–1023.
Article8. Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiqer E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaquir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012; 40(Web Server issue):W597–W603.
Article9. Azevedo MA, Felipe MSS, Maranhão AQ, De Souza MT. Técnicas Básicas em Biologia Molecular. 1st ed. Brasília: Editora Universidade de Brasília;2003. p. 49–72.10. Costa OJ, Batista JA Jr, Silva M, Guimarães MP. Ehrlichia canis infection in dog in Belo Horizonte - Brazil. Arquivos da Escola de Veterinária da UFMG. 1973; 25:199–206.11. Crocquet-Valdes PA, Thirumalapura NR, Ismail N, Yu X, Saito TB, Stevenson HL, Pietzsch CA, Thomas S, Walker DH. Immunization with Ehrlichia p28 outer membrane proteins confers protection in a mouse model of ehrlichiosis. Clin Vaccine Immunol. 2011; 18:2018–2025.
Article12. Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005; 7:504–510.
Article13. Hsieh YC, Lee CC, Tsang CL, Chung YT. Detection and characterization of four novel genotypes of Ehrlichia canis from dogs. Vet Microbiol. 2010; 146:70–75.
Article14. Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999; 9:868–877.
Article15. Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988; 4:181–186.
Article16. Kamani J, Lee CC, Haruna AM, Chung PJ, Weka PR, Chung YT. First detection and molecular characterization of Ehrlichia canis from dogs in Nigeria. Res Vet Sci. 2013; 94:27–32.
Article17. Keysary A, Waner T, Rosner M, Warner CK, Dawson JE, Zass R, Biggie KL, Harrus S. The first isolation, in vitro propagation, and genetic characterization of Ehrlichia canis in Israel. Vet Parasitol. 1996; 62:331–340.
Article18. Labruna MB, McBride JW, Camargo LM, Aguiar DM, Yabsley MJ, Davidson WR, Stromdahl EY, Williamson PC, Stich RW, Long SW, Camargo EP, Walker DH. A preliminary investigation of Ehrlichia species in ticks, humans, dogs and capybaras from Brazil. Vet Parasitol. 2007; 143:189–195.
Article19. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.
Article20. McBride JW, Ndip LM, Popov VL, Walker DH. Identification and functional analysis of an immunoreactive DsbA-like thio-disulfide oxidoreductase of Ehrlichia spp. Infect Immun. 2002; 70:2700–2703.
Article21. McBride JW, Yu XJ, Walker DH. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: a potential serodiagnostic antigen. Clin Diagn Lab Immunol. 1999; 6:392–399.
Article22. McBride JW, Walker DH. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev Vaccines. 2010; 9:1071–1082.
Article23. Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serological survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998; 79:325–339.
Article24. Nazari M, Lim SY, Watanabe M, Sharma RSK, Cheng NABY, Watanabe M. Molecular detection of Ehrlichia canis in dogs in Malaysia. PLoS Negl Trop Dis. 2013; 7:e1982.25. Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998; 66:132–139.
Article26. Rar V, Golovljova I. Anaplasma, Ehrlichia, and "Candidatus Neoehrlichia" bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. 2011; 11:1842–1861.
Article27. Rikihisa Y, Ewing SA, Fox JC. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol. 1994; 32:2107–2112.
Article28. Romero LE, Meneses AI, Salazar L, Jiménez M, Romero JJ, Aguiar DM, Labruna MB, Dolz G. First isolation and molecular characterization of Ehrlichia canis in Costa Rica, Central America. Res Vet Sci. 2011; 91:95–97.
Article29. Santos F, Coppede JS, Pereira AL, Oliveira LP, Roberto PG, Benedetti RB, Zucoloto LB, Lucas F, Sobreira L, Marins M. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto, Brazil. Vet J. 2009; 179:145–148.
Article30. Thomas S, Thirumalapura NR, Crocquet-Valdes PA, Luxon BA, Walker DH. Structure-based vaccines provide protection in a mouse model of ehrlichiosis. PLoS One. 2011; 6:e27981.
Article31. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22:4673–4680.
Article32. Torres HM, Massard CL, de Figueiredo MJ, Ferreira T, Almosny NRP. Isolation and propagation of Ehrlichia canis in DH82 cells as a source of antigens in indirect immunofluorescence test. Revista brasileira de ciência veterinária. 2002; 9:77–82.33. Unver A, Perez M, Orellana N, Huang H, Rikihisa Y. Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks and human in Venezuela. J Clin Microbiol. 2001; 39:2788–2793.
Article34. Vieira RF, Biondo AW, Guimarães AM, Dos Santos AP, Dos Santos RP, Dutra LH, Diniz PP, de Morais HA, Messick JB, Labruna MB, Vidotto O. Ehrlichiosis in Brazil. Rev Bras Parasitol Vet. 2011; 20:1–12.
Article35. Yu XJ, McBride JW, Walker DH. Restriction and expansion of Ehrlichia strain diversity. Vet Parasitol. 2007; 143:337–346.36. Zhang X, Luo T, Keysary A, Baneth AG, Miyashiro S, Strenger C, Waner T, McBride JW. Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains. Clin Vaccine Immunol. 2008; 15:1080–1088.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sequence and phylogenetic analysis of the gp200 protein of Ehrlichia canis from dogs in Taiwan
- Detection and molecular characterization of Hepatozoon canis, Babesia vogeli, Ehrlichia canis, and Anaplasma platys in dogs from Metro Manila, Philippines
- Detection of Tick-Borne Pathogens in the Korean Water Deer (Hydropotes inermis argyropus) from Jeonbuk Province, Korea
- Distribution of Causative Fungi in Home Environment of Parients with Tinea Capitis Caused bu Microsporum Canis
- Isolation and molecular characterizations of canine distemper virus from a naturally infected Korean dog using Vero cells expressing dog signaling lymphocyte activation molecule