J Periodontal Implant Sci.
2012 Aug;42(4):113-118. 10.5051/jpis.2012.42.4.113.
The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. guy@snu.ac.kr
- 2Dental Regenerative Biotechnology, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 1783646
- DOI: http://doi.org/10.5051/jpis.2012.42.4.113
Abstract
- PURPOSE
The aim of this study was to investigate the effects of synthetic fibronectin (FN) fragments, including fibrin binding sites from amino-terminal FN fragments containing type I repeats 1 to 5, on osteoblast-like cell activity.
METHODS
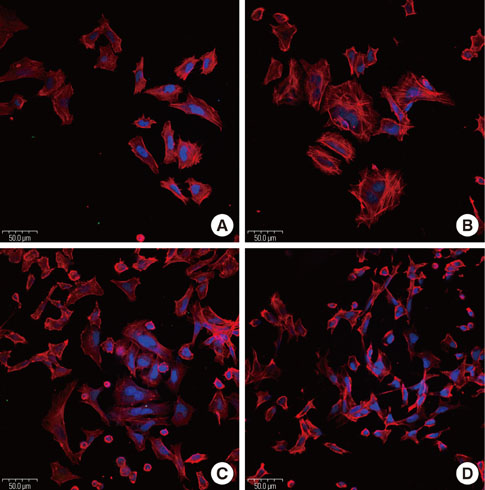
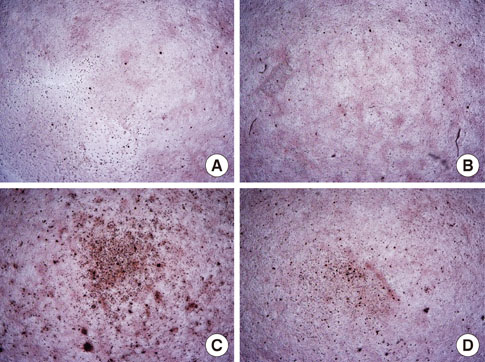
Oligopeptides ranging from 9 to 20 amino acids, designated FF1, FF3, and FF5, were synthesized by a solid-phase peptide synthesizing system, and we investigated the effects of these peptides on cell attachment and extent of mineralization using confocal microscopy, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, and Alizarin red S staining.
RESULTS
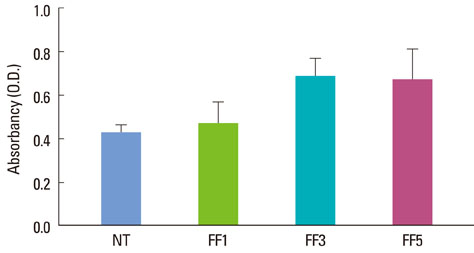
FF3 and FF5 peptides increased the number of attached human osteoblastic cells, and FF3 administration led to prominent cell spreading. Mineralization was increased in FF3 and FF5 compared to FF1 and the untreated control.
CONCLUSIONS
Taken together, it can be concluded that the fibrin-binding oligopeptides FF3 and FF5 enhanced cell attachment and mineralization on osteoblast-like cells. These results indicate that FF3 and FF5 have the potential to increase osteoblast-like cell activity. Performing an in vivo study may provide further possibilities for surface modification of biomimetic peptides to enhance osteogenesis, thus improving the regeneration of destroyed alveolar bone.
Keyword
MeSH Terms
Figure
Reference
-
1. Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone. 1999. 25:95–96.
Article2. Embery G, Waddington RJ, Hall RC, Last KS. Connective tissue elements as diagnostic aids in periodontology. Periodontol 2000. 2000. 24:193–214.
Article3. Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004. 199:174–180.
Article4. Mosher DF. Fibronectin. 1989. San Diego: Academic Press.5. Garcia AJ, Ducheyne P, Boettiger D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J Biomed Mater Res. 1998. 40:48–56.
Article6. Ding HT, Wang CG, Zhang TL, Wang K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem. 2006. 99:1343–1352.
Article7. Majmudar G, Bole D, Goldstein SA, Bonadio J. Bone cell culture in a three-dimensional polymer bead stabilizes the differentiated phenotype and provides evidence that osteoblastic cells synthesize type III collagen and fibronectin. J Bone Miner Res. 1991. 6:869–881.
Article8. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002. 115(Pt 20):3861–3863.
Article9. Mardon HJ, Grant KE. The role of the ninth and tenth type III domains of human fibronectin in cell adhesion. FEBS Lett. 1994. 340:197–201.
Article10. Redick SD, Settles DL, Briscoe G, Erickson HP. Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J Cell Biol. 2000. 149:521–527.
Article11. Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998. 18:1363–1370.12. Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997. 272:24999–25005.
Article13. Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980. 19:517–525.
Article14. Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996. 88:158–166.
Article15. Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997. 110(Pt 7):861–870.
Article16. Clark RA, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol. 2004. 122:266–277.
Article17. Knox P, Crooks S, Rimmer CS. Role of fibronectin in the migration of fibroblasts into plasma clots. J Cell Biol. 1986. 102:2318–2323.
Article18. Rostagno AA, Schwarzbauer JE, Gold LI. Comparison of the fibrin-binding activities in the N- and C-termini of fibronectin. Biochem J. 1999. 338(Pt 2):375–386.
Article19. Matsuka YV, Medved LV, Brew SA, Ingham KC. The NH2-terminal fibrin-binding site of fibronectin is formed by interacting fourth and fifth finger domains. Studies with recombinant finger fragments expressed in Escherichia coli. J Biol Chem. 1994. 269:9539–9546.
Article20. Garcia-Pardo A, Pearlstein E, Frangione B. Primary structure of human plasma fibronectin. Characterization of a 31,000-dalton fragment from the COOH-terminal region containing a free sulfhydryl group and a fibrin-binding site. J Biol Chem. 1985. 260:10320–10325.
Article21. Cutler SM, Garcia AJ. Engineering cell adhesive surfaces that direct integrin alpha5beta1 binding using a recombinant fragment of fibronectin. Biomaterials. 2003. 24:1759–1770.
Article22. Kim TI, Jang JH, Chung CP, Ku Y. Fibronectin fragment promotes osteoblast-associated gene expression and biological activity of human osteoblast-like cell. Biotechnol Lett. 2003. 25:2007–2011.
Article23. Kim TI, Jang JH, Lee YM, Rhyu IC, Chung CP, Han SB, et al. Biomimetic approach on human periodontal ligament cells using synthetic oligopeptides. J Periodontol. 2004. 75:925–932.
Article24. Ku Y, Chung CP, Jang JH. The effect of the surface modification of titanium using a recombinant fragment of fibronectin and vitronectin on cell behavior. Biomaterials. 2005. 26:5153–5157.
Article25. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.
Article26. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007. 7:26.
Article27. Clover J, Gowen M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone. 1994. 15:585–591.
Article28. Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003. 121:695–705.
Article29. Purohit A, Flanagan AM, Reed MJ. Estrogen synthesis by osteoblast cell lines. Endocrinology. 1992. 131:2027–2029.
Article30. Kinpara K, Mogi M, Kuzushima M, Togari A. Osteoclast differentiation factor in human osteosarcoma cell line. J Immunoassay. 2000. 21:327–340.
Article31. Rostagno A, Williams MJ, Baron M, Campbell ID, Gold LI. Further characterization of the NH2-terminal fibrin-binding site on fibronectin. J Biol Chem. 1994. 269:31938–31945.
Article32. Williams MJ, Phan I, Harvey TS, Rostagno A, Gold LI, Campbell ID. Solution structure of a pair of fibronectin type 1 modules with fibrin binding activity. J Mol Biol. 1994. 235:1302–1311.
Article33. Blystone SD, Weston LK, Kaplan JE. Fibronectin dependent macrophage fibrin binding. Blood. 1991. 78:2900–2907.
Article34. Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998. 18:1363–1370.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The biologic effect of fibrin-binding synthetic oligopeptide on periodontal ligament cells
- Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects
- The biological effects of fibronectin typeIII 7-10 to MC3T3-E1 osteoblast
- Effects of H2O2 Derived Hydroxyl Radicals Treated Fibronectin on Rat Calvarial Osteoblast
- Biological Effects of Fibronectin Type III 10 domain on Human Osteoblast-like cells