J Korean Med Sci.
2008 Oct;23(5):825-832. 10.3346/jkms.2008.23.5.825.
Impaired Delta NP63 Expression is Associated with Poor Tumor Development in Transitional Cell Carcinoma of the Bladder
- Affiliations
-
- 1Department of Urological Surgery, the First Affiliated Hospital, ChongQing Medical University, ChongQing, China. hyf028@163.com
- 2Department of Respiratory Medicine, The Children's Hospital, ChongQing Medical University, ChongQing, China.
- 3Faculty of Laboratory Medicine, ChongQing Medical University, ChongQing, China.
- KMID: 1783068
- DOI: http://doi.org/10.3346/jkms.2008.23.5.825
Abstract
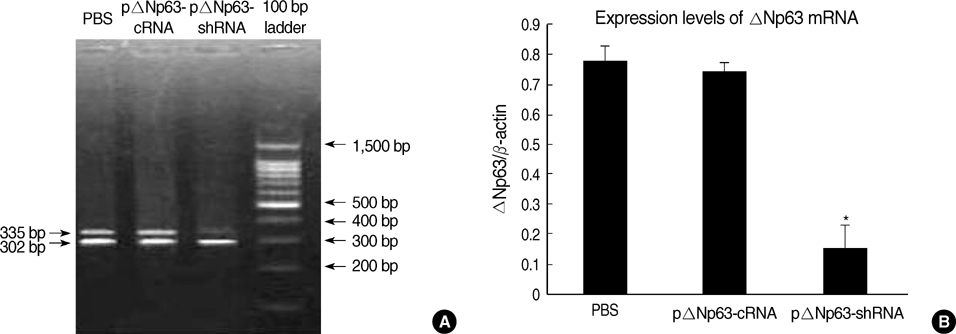
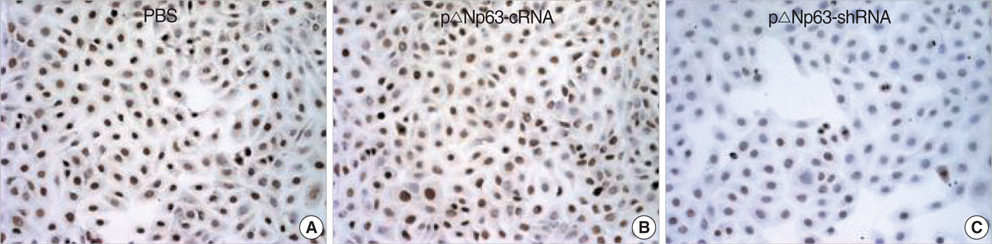
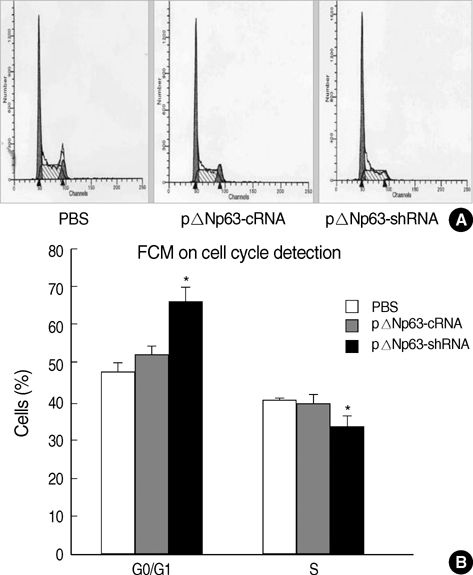
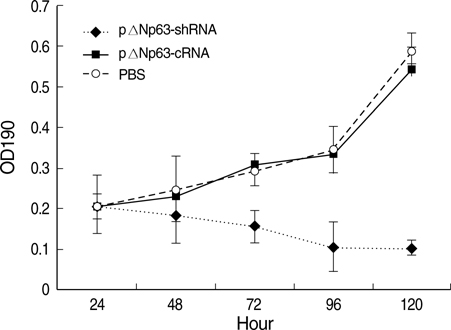
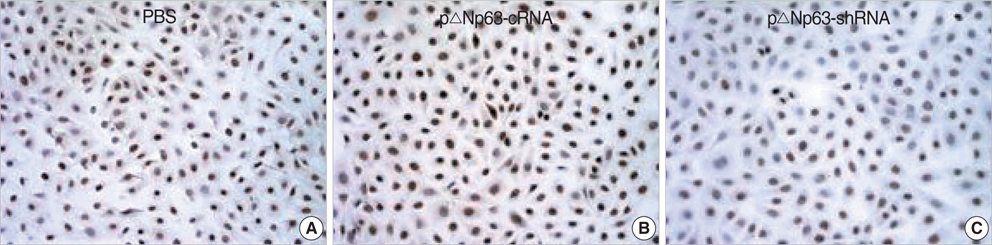
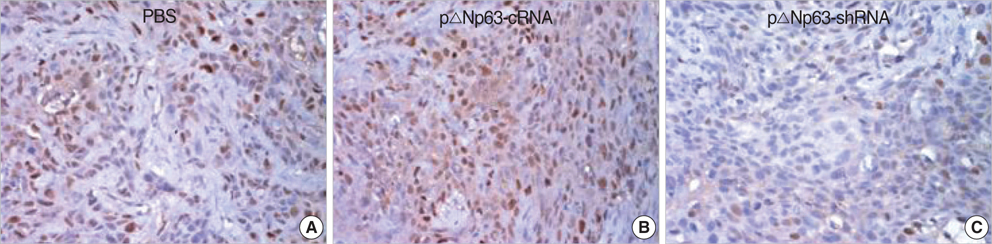
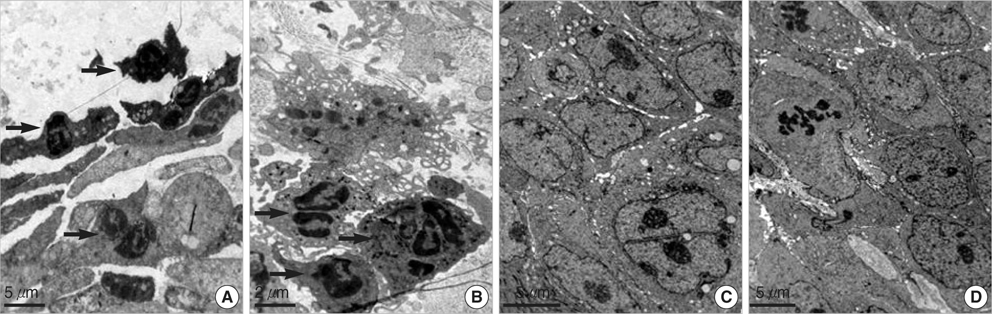
- The oncogenic isoform of the p63 protein, delta NP63, plays an important role in the pathogenesis of many epithelial carcinomas, and emerging evidences suggest that delta NP63 is a promising drug target. However, the functions of delta NP63 in transitional cell carcinoma of bladder (TCCB) are poorly defined. In this study, a delta NP63 shRNA expression vector was transfected into TCCB cell line 5637 and cell cycling, cell proliferation and protein expression were assessed by flow cytometry and 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-dimethyl tetrazolium bromide (MTT) assay, and immunohistochemistry, respectively. The delta NP63 shRNA expression vector was also injected into 5637 cell xenograft tumors in nude mice, and tumor size was measured, tumor tissue morphology was assessed by immunohistopathology and transmission electron microscopy. In the in vitro study, delta NP63 shRNA transfection caused successful delta NP63 gene silencing and resulted in significant arrest of cell cycling and cellular proliferation (p<0.05) as well as cyclin D1 expression. In the nude mouse xenograft model, delta NP63 shRNA greatly inhibited tumor growth, induced tumor cell apoptosis (p<0.05) and resulted in cyclin D1 downregulation. Our data suggest that delta NP63 may play an oncogenic role in TCCB progression through promoting cell survival and proliferation. Intratumoral administration of delta NP63-specific shRNA suppressed tumor delta NP63 expression and cellular proliferation while promoted tumor cellular apoptosis, and therefore inhibited tumor growth and improved survival of xenograft-bearing mice, which was not accompanied by significant signs of systemic toxicity.
MeSH Terms
-
Animals
Carcinoma, Transitional Cell/*genetics/metabolism
Cell Line, Tumor
Cell Proliferation
Cyclin D1/biosynthesis
Disease Progression
Female
Humans
Mice
Mice, Nude
Microscopy, Electron, Transmission
Models, Biological
Neoplasm Transplantation
Trans-Activators/*biosynthesis/*physiology
Tumor Suppressor Proteins/*biosynthesis/*physiology
Urinary Bladder Neoplasms/*genetics/metabolism
Figure
Reference
-
1. Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997. 90:809–819.
Article2. Schmale H, Bamberger C. A novel protein with strong homology to the tumor suppressor p53. Oncogene. 1997. 15:1363–1367.
Article3. Finlan LE, Hupp TR. p63: the phantom of the tumor suppressor. Cell Cycle. 2007. 6:1062–1071.
Article4. Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007. 26:5169–5183.
Article5. Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998. 2:305–316.
Article6. Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000. 1:199–207.
Article7. Augustin M, Bamberger C, Paul D, Schmale H. Cloning and chromosomal mapping of the human p53-related KET gene to chromosome 3q27 and its murine homolog ket to mouse chromosome 16. Mamm Genome. 1998. 9:899–902.
Article8. Osada M, Ohba M, Kawahara C, Ishioka C, Kanamaru R, Katoh I, Ikawa Y, Nimura Y, Nakagawara A, Obinata M, Ikawa S. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat Med. 1998. 4:839–843.
Article9. Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, Dong SM, Guo Z, Benoit N, Cohen Y, Rechthand P, Califano J, Moon CS, Ratovitski E, Jen J, Sidransky D, Trink B. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003. May. 15. 63:2351–2357.10. Ratovitski EA, Patturajan M, Hibi K, Trink B, Yamaguchi K, Sidransky D. p53 associates with and targets delta Np63 into a protein degradation pathway. Proc Natl Acad Sci USA. 2001. 98:1817–1822.11. Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000. 97:5462–5467.
Article12. Patturajan M, Nomoto S, Sommer M, Fomenkov A, Hibi K, Zangen R, Poliak N, Califano J, Trink B, Ratovitski E, Sidransky D. Delta Np63 induces beta-catenin nuclear accumulation and signaling. Cancer Cell. 2002. 1:369–379.13. Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999. 113:1099–1105.
Article14. Choi HR, Batsakis JG, Zhan F, Sturgis E, Luna MA, El-Naggar AK. Differential expression of p53 gene family members p63 and p73 in head and neck squamous tumorigenesis. Hum Pathol. 2002. 33:158–164.
Article15. Ratovitski EA, Patturajan M, Hibi K, Trink B, Yamaguchi K, Sidransky D. p53 associates with and targets delta Np63 into a protein degradation pathway. Proc Natl Acad Sci USA. 2001. 98:1817–1822.16. Park CK, Oh YH. Expression of p63 in reactive hyperplasias and malignant lymphomas. J Korean Med Sci. 2005. 20:752–758.
Article17. Dohn M, Zhang S, Chen X. p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001. 20:3193–3205.18. Urist MJ, Di Como CJ, Lu ML, Charytonowicz E, Verbel D, Crum CP, Ince TA, McKeon FD, Cordon-Cardo C. Loss of p63 Expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002. 161:1199–1206.
Article19. Kim VN. RNA interference in functional genomics and medicine. J Korean Med Sci. 2003. 18:309–318.
Article20. Filleur S, Courtin A, Ait-Si-Ali S, Guglielmi J, Merle C, Harel-Bellan A, Clézardin P, Cabon F. SiRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth. Cancer Res. 2003. 63:3919–3922.21. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002. 296:550–553.
Article22. Wilda M, Fuchs U, Wossmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Oncogene. 2002. 21:5716–5724.
Article23. Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002. 8:494–501.24. Harmes DC, Bresnick E, Lubin EA, Watson JK, Heim KE, Curtin JC, Suskind AM, Lamb J, DiRenzo J. Positive and negative regulation of deltaN-p63 promoter activity by p53 and deltaN-p63-alpha contributes to differential regulation of p53 target genes. Oncogene. 2003. 22:7607–7616.25. Reznikoff CA, Sarkar S, Julicher KP, Burger MS, Puthenveettil JA, Jarrard DF, Newton MA. Genetic alterations and biological pathways in human bladder cancer pathogenesis. Urol Oncol. 2000. 5:191–203.
Article26. Lee CC, Yamamoto S, Morimura K, Wanibuchi H, Nishisaka N, Ikemoto S, Nakatani T, Wada S, Kishimoto T, Fukushima S. Significance of cyclin D1 overexpression in transitional cell carcinomas of the urinary bladder and its correlation with histopathologic features. Cancer. 1997. 79:780–789.
Article27. Shin KY, Kong G, Kim WS, Lee TY, Woo YN, Lee JD. Overexpression of cyclin D1 correlates with early recurrence in superficial bladder cancers. Br J Cancer. 1997. 75:1788–1792.
Article28. Suwa Y, Takano Y, Iki M, Takeda M, Asakura T, Noguchi S, Masuda M. Cyclin D1 protein overexpression is related to tumor differentiation, but not to tumor progression or proliferative activity, in transitional cell carcinoma of the bladder. J Urol. 1998. 160:897–900.
Article29. Sgambato A, Migaldi M, Faraglia B, De Aloysio G, Ferrari P, Ardito R, De Gaetani C, Capelli G, Cittadini A, Trentini GP. Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer. 2002. 97:671–678.
Article30. Wagner U, Süess K, Luginbühl T, Schmid U, Ackermann D, Zellweger T, Maurer R, Alund G, Knönagel H, Rist M, Jordan P, Moch H, Mihatsch MJ, Gasser TC, Sauter G. Cyclin D1 overexpression lacks prognostic significance in superficial urinary bladder cancer. J Pathol. 1999. 188:44–50.
Article31. Oya M, Schmidt B, Schmitz-Dräger BJ, Schulz WA. Expression of G1-->S transition regulatory molecules in human urothelial cancer. Jpn J Cancer Res. 1998. 89:719–726.32. Monica SM, George F. Mendelson J, editor. Regulation of the cell cycle. The molecular basis of cancer. 2001. 2nd ed. W. B. Saunders Company;10–17.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of p63 and its Isoform, deltaNp63, in Non-Small Cell Lung Carcinoma
- Correlation between Histopathologic Grade, Stage, and Degree of EGFR Expression in Transitional Cell Carcinoma of the Urinary Bladder
- A Case of Micropapillary Transitional Cell Carcinoma of the Urinary Bladder
- Papillary Transitional Cell Carcinoma of the Bladder: Report of a Case
- Primary Ovarian Transitional Cell Carcinoma