J Korean Med Sci.
2009 Oct;24(5):853-859. 10.3346/jkms.2009.24.5.853.
Differences in Circulating Dendritic Cell Subtypes in Pregnant Women, Cord Blood and Healthy Adult Women
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 2Seoul Metropolitan Public Cord Blood Bank, Seoul, Korea.
- 3Department of Family Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 4Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, College of Medicine, Konkuk University, Seoul, Korea. eysongmd@hanmail.net
- 6Department of Biomedical Science and Technology, College of Medicine, Konkuk University, Seoul, Korea.
- 7Department of Respiratory Medicine, College of Medicine, Konkuk University, Seoul, Korea.
- KMID: 1782006
- DOI: http://doi.org/10.3346/jkms.2009.24.5.853
Abstract
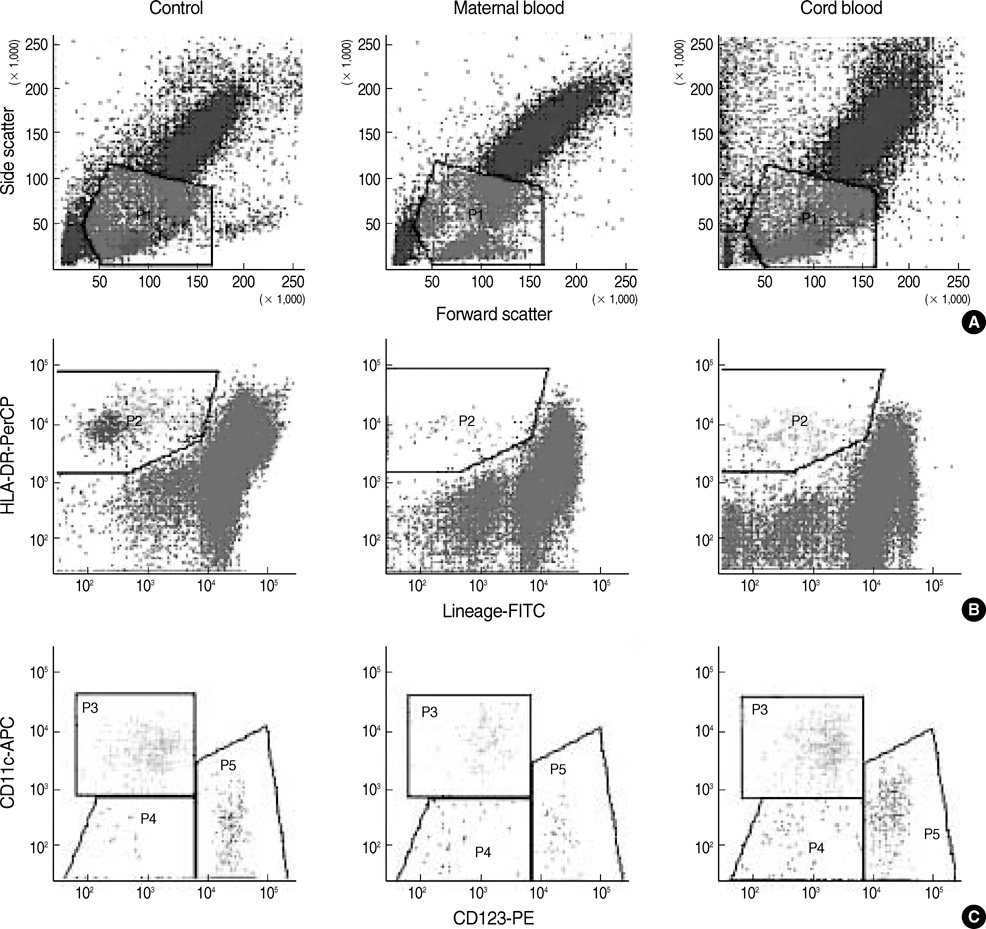
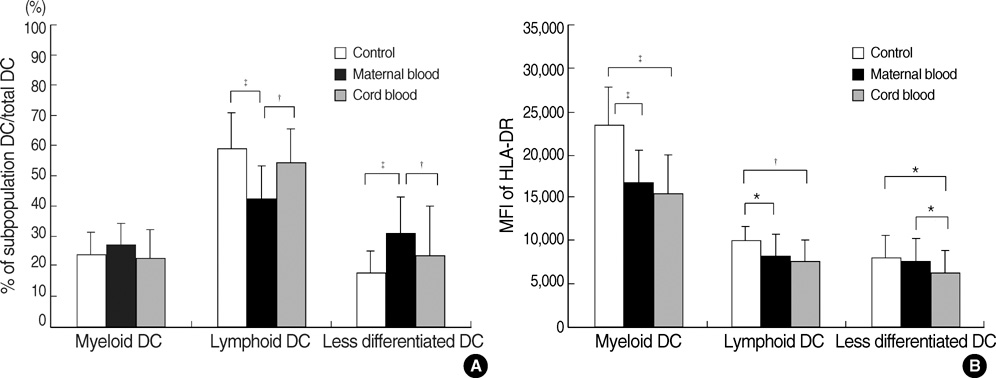
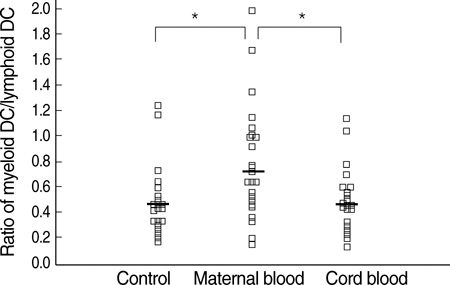
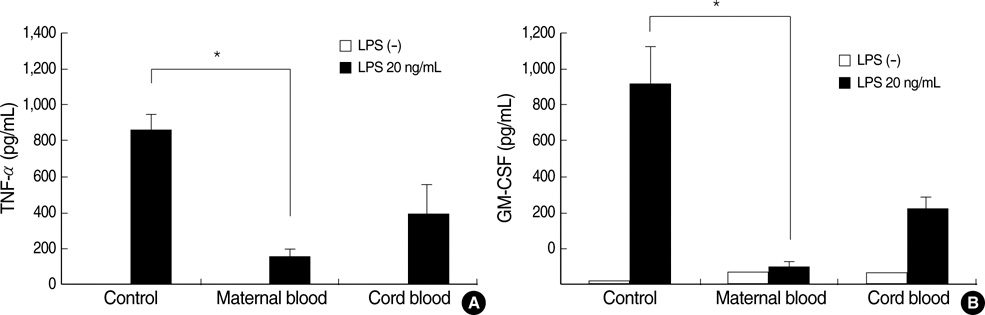
- Different subtypes of dendritic cells (DC) influence the differentiation of naive T lymphocytes into T helper type 1 (Th1) and Th2 effector cells. We evaluated the percentages of DC subtypes in peripheral blood from pregnant women (maternal blood) and their cord blood compared to the peripheral blood of healthy non pregnant women (control). Circulating DC were identified by flow cytometry as lineage (CD3, CD14, CD16, CD19, CD20, and CD56)-negative and HLA-DR-positive cells. Subtypes of DC were further characterized as myeloid DC (CD11c+/CD123+/-), lymphoid DC (CD11c-/CD123+++) and less differentiated DC (CD11c-/CD123+/-). The frequency of DC out of all nucleated cells was significantly lower in maternal blood than in control (P<0.001). The ratio of myeloid DC/lymphoid DC was significantly higher in maternal blood than in control (P<0.01). HLA-DR expressions of myeloid DC as mean fluorescence intensity (MFI) were significantly less in maternal blood and in cord blood than in control (P<0.001, respectively). The DC differentiation factors, TNF-alpha and GM-CSF, released from mononuclear cells after lipopolysaccharide stimulation were significantly lower in maternal blood than in control (P<0.01). The distribution of DC subtypes was different in maternal and cord blood from those of non-pregnant women. Their role during pregnancy remains to be determined.
Keyword
MeSH Terms
-
Adult
Cell Differentiation
Dendritic Cells/*classification/cytology/immunology
Female
Fetal Blood/cytology/*immunology
Flow Cytometry
Granulocyte-Macrophage Colony-Stimulating Factor/metabolism
HLA-DR Antigens/metabolism
Humans
Lipopolysaccharides/pharmacology
Lymphocyte Activation
Pregnancy
T-Lymphocytes/cytology/immunology
Th1 Cells/cytology/immunology
Th2 Cells/cytology/immunology
Tumor Necrosis Factor-alpha/metabolism
Figure
Reference
-
1. Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003. 21:3352–3357.
Article2. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989. 7:145–173.
Article3. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993. 14:353–356.
Article4. Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1-and Th2-type cytokines. Immunol Res. 1996. 15:141–150.5. Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999. 20:330–335.
Article6. Liu E, Tu W, Law HK, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001. 113:240–246.
Article7. Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002. 128:118–123.
Article8. Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003. 111:675–697.
Article9. Adams S, O'Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005. 25:177–188.
Article10. Uchida Y, Kurasawa K, Nakajima H, Nakagawa N, Tanabe E, Sueishi M, Saito Y, Iwamoto I. Increase of dendritic cells of type 2 (DC2) by altered response to IL-4 in atopic patients. J Allergy Clin Immunol. 2001. 108:1005–1011.
Article11. Summers KL, Hock BD, McKenzie JL, Hart DN. Phenotypic characterization of five dendritic cell subsets in human tonsils. Am J Pathol. 2001. 159:285–295.
Article12. Hagendorens MM, Ebo DG, Schuerwegh AJ, Huybrechs A, Van Bever HP, Bridts CH, De Clerck LS, Stevens WJ. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin Exp Allergy. 2003. 33:633–639.
Article13. Sorg RV, Kogler G, Wernet P. Identification of cord blood dendritic cells as an immature CD11c- population. Blood. 1999. 93:2302–2307.
Article14. Borras FE, Matthews NC, Lowdell MW, Navarrete CV. Identification of both myeloid CD11c+ and lymphoid CD11c- dendritic cell subsets in cord blood. Br J Haematol. 2001. 113:925–931.15. Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood and peripheral blood enumerated by a novel method. Stem Cells. 2003. 21:296–303.
Article16. Schibler KR, Georgelas A, Rigaa A. Developmental biology of the dendritic cell system. Acta Paediatr. 2002. 91:9–16.
Article17. Darmochwal-Kolarz D, Rolinski J, Buczkowski J, Tabarkiewicz J, Leszczynska-Gorzelak B, Zych I, Oleszczuk J. CD1c (+) immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol Lett. 2004. 91:71–74.18. Ueda Y, Hagihara M, Okamoto A, Higuchi A, Tanabe A, Hirabayashi K, Izumi S, Makino T, Kato S, Hotta T. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol. 2003. 64:1144–1151.
Article19. Aldebert D, Diallo M, Niang M, Sarr D, Cisse C, Moreau JC, Jambou R. Differences in circulating dendritic cell subtypes in peripheral, placental and cord blood in African pregnant women. J Reprod Immunol. 2007. 73:11–19.
Article20. Almeida J, Bueno C, Alguero MC, Sanchez ML, Canizo MC, Fernandez ME, Vaquero JM, Laso FJ, Escribano L, San Miguel JF, Orfao A. Extensive characterization of the immunophenotype and the pattern of cytokine production by distinct subpopulations of normal human peripheral blood dendritic cells. Clin Exp Immunol. 1999. 118:392–401.21. Adams KM, Yan Z, Stevens AM, Nelson JL. The changing maternal "self" hypothesis: a mechanism for maternal tolerance of the fetus. Placenta. 2007. 28:378–382.
Article22. Gerrits JH, Athanassopoulos P, Vaessen LM, Klepper M, Weimar W, van Besouw NM. Peripheral blood manipulation significantly affects the result of dendritic cell monitoring. Transpl Immunol. 2007. 17:169–177.
Article23. Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004. 10:7260–7269.
Article24. Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000. 192:219–226.25. Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive potent TH1 polarization. Nat Immunol. 2000. 1:305–310.26. Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007. 123:40–49.
Article27. Pinzon-Charry A, Maxwell T, Prato S, Furnival C, Schmidt C, Lopez JA. HLA-DR+ immature cells exhibit reduced antigen-presenting cell function but respond to CD40 stimulation. Neoplasia. 2005. 7:1123–1132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the Values of Total Serum Cholesterol in Healthy Non-Pregnant and Pregnant Women
- The Effects of General , Spinal and Epidural Anesthesia on Maternal and Umbilical Cord Plasma beta- endorphin Level
- The Effect of Fish Consumption on Blood Mercury Levels of Pregnant Women
- Lipid Peroxidation and Antioxidant Activity in Pregnant Women with Pregnancy-Induced Hypertension
- Overall health and drinking behavior among pregnant and breastfeeding women in Korea