J Korean Med Sci.
2006 Oct;21(5):904-910. 10.3346/jkms.2006.21.5.904.
Increased Expression of Heat Shock Protein 72 Protects Renal Proximal Tubular Cells from Gentamicin-induced Injury
- Affiliations
-
- 1Department of Pharmacology, School of Pharmacy, Fourth Military Medical University, Xi'an, Shaanxi, 710032, China. qbmei@fmmu.edu.cn
- KMID: 1781913
- DOI: http://doi.org/10.3346/jkms.2006.21.5.904
Abstract
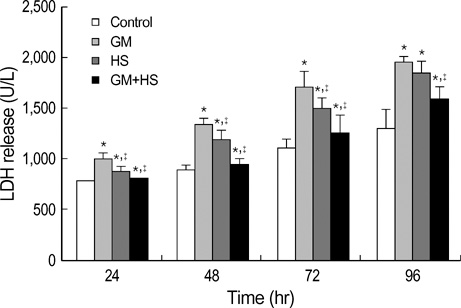
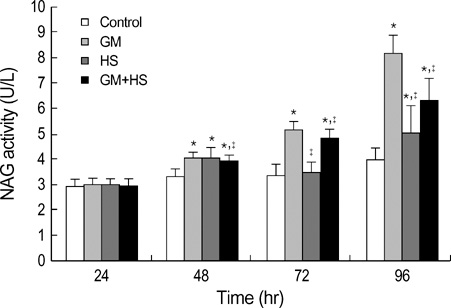
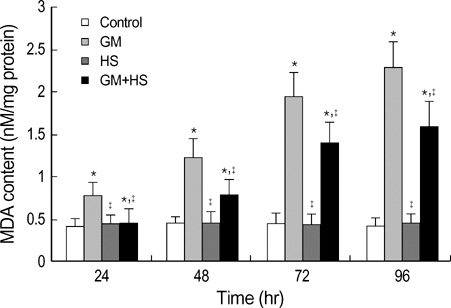
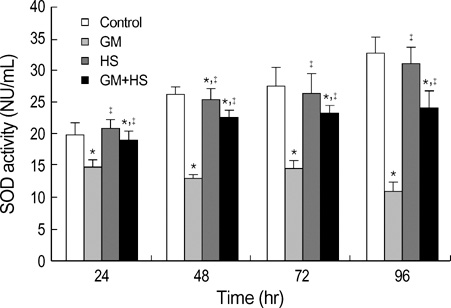
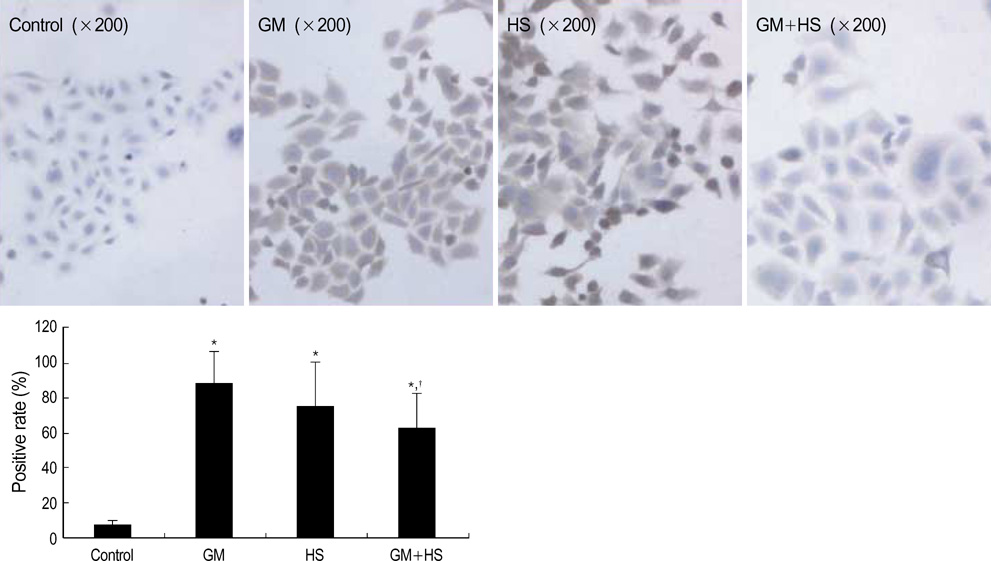
- The nephrotoxicity of gentamicin (GM) has been widely recognized. Heat shock protein 72 (HSP72) has been reported to be a cytoprotectant. However, its cytoprotective effect against GM induced kidney injury has not yet been studied. In this study, we investigated the cytoprotective effect of HSP72 on GM-induced nephrotoxicity in vitro. Human Kidney tubular cell line, HK-2 cells were divided into four groups: control group, GM group (cells incubated with GM only), heat shock (HS) group (cells incubated at 43 degrees C for 30 min), and GM plus HS group, respectively. Lactate dehydrogenanse (LDH) release increased time-dependently from 24 hr to 96 hr compared to the data of cells treated with GM only. Results of NAG activities, superoxide dismutase (SOD) activities and malondialdehyde (MDA) content were similar to that of the LDH release. The amount of HSP72 positive cells increased significartly at 72 hr after cells were treated with GM only. Both HSP72 protein and gene expression increased significantly at 72 hr when cells were treated with GM. On the other hand, HS induced HSP72 expression markedly. Pretreatment of HS inhibited HK-2 cells from GM-induced injury. It could reduce LDH release and NAG activity. HS also increased SOD activity, and decreased MDA content when cells were damaged by GM. These findings suggested that HS may protect kidney cells from GM-induced injury. Pre-induction of HSP72 may provide therapeutic strategies for nephrotoxicity induced by GM.
Keyword
MeSH Terms
Figure
Reference
-
1. Atessahin A, Karahan I, Yilmaz S, Çeribasi AO, Princci I. The effect of manganese chloride on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2003. 48:637–642.
Article2. Pedraza-Chaverrí J, Maldonado PD, Barrera D, Cerón A, Medina-Campos ON, Hernández-Pando R. Protective effect of diallyl sulfide on oxidative stress and nephrotoxicity induced by gentamicin in rats. Mol and Cell Biochem. 2003. 254:125–130.3. Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernandez-Pando R, Pedraza-Chaverr JE. Antioxidant S-allylcysteine prevents gentamicin-induced oxidantive stress and renal damage. Free Rad Biol Med. 2003. 35:317–324.4. Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999. 21:433–442.
Article5. Chen Y, Ross BM, Currie RW. Heat shock treatment protects against angiotensin II-induced hypertension and inflammation in aorta. Cell Stress Chaperones. 2004. 9:99–107.
Article6. Mikami K, Otaka M, Goto T, Miura K, Ohshima S, Yoneyama K, Lin JG, Watanabe D, Segawa D, Kataoka E, Odashima M, Watanabe S. Induction of a 72-kDa heat shock protein and protection against lipopolysaccharide-induced liver injury in cirrhotic rats. J Gastroenterol Hepatol. 2004. 19:884–890.
Article7. Zhou H, Kato A, Yasuda H, Odamaki M, Itoh H, Hishida A. The induction of heat shock protein-72 attenuates cisplatin-induced acute renal failure in rats. Pflugers Arch. 2003. 446:116–124.
Article8. Suzuki S, Maruyama S, Sato W, Morita Y, Sato F, Miki Y, Kato S, Katsuno M, Sobue G, Yuzawa Y, Matsuo S. Geranylgeranylacetone ameliorates ischemic acute renal failure via induction of Hsp70. Kidney Int. 2005. 67:2210–2220.
Article9. Chen Y, Ross BM, Currie RW. Heat shock treatment protects against angiotensin II-induced hypertension and inflammation in aorta. Cell Stress Chaperones. 2004. 9:99–107.
Article10. Hadley M, Draper HH. Isolation of a guanine-malondialdehyde adduct from rat and human urine. Lipids. 1990. 25:82–85.
Article11. Mao H, Wang Y, Li Z, Ruchalski KL, Yu X, Schwartz JH, Borkan SC. Hsp72 interacts with paxillin and facilitates the reassembly of focal adhesions during recovery from ATP depletion. J Biol Chem. 2004. 279:15472–15480.
Article12. Li CY, Suardet L, Little JB. Potential role of WAF1/Cip1/p21 as a mediator of TGF-β cytoinhibitory effect. J Biol Chem. 1995. 270:4971–4974.
Article13. Fashola TO, Obatomi DK, Plummer DT. The combined effect of cyclosporine a and gentamicin on enzymuria in the Sprague-Dawley rat. Ren Fail. 2000. 22:283–295.
Article14. Fox RB. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984. 74:1456–1467.
Article15. Emami A, Schwartz JH, Borkan SC. Transient ischemia or heat stress induces a cytoprotectant protein in rat kidney. Am J Physiol. 1991. 260:F479–F485.
Article16. Morita K, Wakui H, Komatsuda A, Ohtani H, Miura AB, Itoh H, Tashima Y. Induction of heat-shock proteins HSP73 and HSP90 in rat kidneys after ischemia. Ren Fail. 1995. 17:405–419.
Article17. Komatsuda A, Wakui H, Satoh K, Yasuda T, Imai H, Nakamoto Y, Miura AB, Itoh H, Tashima Y. Altered localization of 73-kilodalton heat-shock protein in rat kidneys with gentamicin-induced acute tubular injury. Lab Invest. 1993. 68:687–695.18. Satoh K, Wakui H, Komatsuda A, Nakamoto Y, Miura AB, Itoh H, Tashima Y. Induction and altered localization of 90-kDa heat-shock protein in rat kidneys with cisplatin-induced acute renal failure. Ren Fail. 1994. 16:313–323.
Article19. Turman MA, Rosenfeld SL. Heat shock protein 70 overexpression protects LLC-PK1 tubular cells from heat shock but not hypoxia. Kidney Int. 1999. 55:189–197.
Article20. Komatsuda A, Wakui H, Oyama Y, Imai H, Miura AB, Itoh H, Tashima Y. Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol Dial Transplant. 1999. 14:1385–1390.
Article21. Abe T, Gotoh S, Higashi K. Higher induction of heat shock protein 72 by heat stress in cisplatin-resistant than in cisplatin-sensitive cancer cells. Biochim Biophys Acta. 1999. 1445:123–133.
Article22. Takumida M, Anniko M. Heat shock protein 70 delays gentamicin-induced vestibular hair cell death. Acta Otolaryngol. 2005. 125:23–28.
Article23. Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di PR, Britti D, De SA, Pierpaoli S, Caputi A, Masini E, Salvemini D. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2002. 450:67–76.
Article24. Sha SH, Schacht J. Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic Biol Med. 1999. 26:341–347.
Article25. Sandhya P, Varalakshmi P. Effect of lipoic acid administration on gentamicin-induced lipid peroxidation in rats. J Appl Toxicol. 1997. 17:405–408.
Article26. Erdem A, Gundogan NU, Usubutun A, Kilinc K, Erdem SR, Kara A, Bozkurt A. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant. 2000. 15:1175–1182.
Article27. Ozbek E, Turkoz Y, Sahna E, Ozugurlu F, Mizrak B, Ozbek M. Melatonin administration prevents the nephrotoxicity induced by gentamicin. BJU Int. 2000. 85:742–746.28. Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2001. 5:267–287.
Article29. Kidwell DT, McKeown JW, Grider JS, McCombs GB, Ott CE, Jackson BA. Acute effects of gentamicin on thick ascending limb function in the rat. Eur J Pharmacol. 1994. 270:97–103.
Article30. Fukuda Y, Malmborg AS, Aperia A. Gentamicin inhibition of Na+, K(+)-ATPase in rat kidney cells. Acta Physiol Scand. 1991. 141:27–34.
Article31. Jesse R, Kristopher TK, Paola de los H, Norma V, Patricia M, Frederick HW, Steven CH, Ignacio G, Gerardo G, Richard PL. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Clcotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005. 102:16777–16782.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Heat Shock Protein 27 and Apoptosis in Renal Cell Carcinomas
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Heat Shock Protein Induction By An Infrared Warm Compression Device
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis