J Korean Med Sci.
2006 Oct;21(5):897-903. 10.3346/jkms.2006.21.5.897.
Prophylactic Low-dose Heparin or Prostaglandin E1 may Prevent Severe Veno-occlusive Disease of the Liver after Allogeneic Hematopoietic Stem Cell Transplantation in Korean Children
- Affiliations
-
- 1Division of Hematology/Oncology/Bone Marrow Transplantation, Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hojim@amc.seoul.kr
- 2Pediatric Hematology-Oncology Center, Korean National Cancer Center, Goyang, Korea.
- KMID: 1781912
- DOI: http://doi.org/10.3346/jkms.2006.21.5.897
Abstract
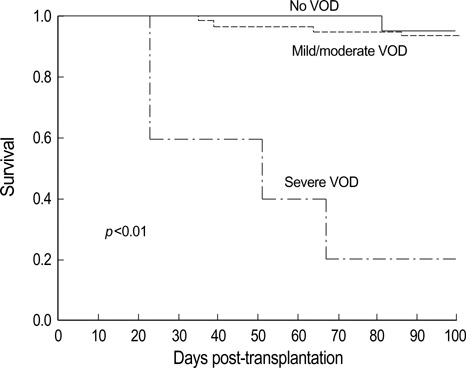
- Studies investigating the effect of prophylactic drugs on hepatic veno-occlusive disease (VOD) development are rare in children that have undergone allogeneic hematopoietic stem cell transplantation (HSCT). This study examined risk factors for VOD, the effect of prophylactic low-dose heparin or lipo-prostaglandin E1 (lipo-PGE1) and the survival rate at day +100 in children undergoing allogeneic HSCT. Eighty five children underwent HSCT between June 1997 and September 2004. Patients were diagnosed and classified as having mild, moderate or severe VOD according to Seattle clinical criteria. Among 85 patients, 25 (29%) developed VOD. VOD occurred more frequently in patients receiving busulfan-based conditioning (24/65, 37%) than in those receiving TBI-based (1/10, 10%) or other (0/10, 0%) regimens (p<0.05). The incidence of VOD was lower in patients with non-malignant disease compared to those with malignant disease (p<0.05). Survival at day +100 for non-VOD patients was better than that for VOD patients (92% vs. 76%, p<0.05). No patients receiving prophylactic heparin or lipo-PGE1 were found to develop severe VOD, whereas 5 of 35 patients not receiving such prophylaxis developed severe VOD. Given severe VOD is associated with a high mortality rate, this study indicates that prophylactic heparin or lipo-PGE1 may decrease mortality in children undergoing HSCT.
Keyword
MeSH Terms
Figure
Reference
-
1. McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993. 118:255–267.
Article2. Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund H, Bandini G, Esperou GB, Russell J, Rubia J, Girolamo GD, Demuynck H, Hartmann O, Clausen J, Ruutu T, Leblond V, Iriondo A, Bosi A, Ben-Bassat I, Koza V, Gratwohl A, Apperley JF. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. Blood. 1998. 92:3599–3604.3. Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987. 44:778–783.
Article4. Ayash LJ, Hunt M, Antman K, Nadler L, Wheeler C, Takvorian T, Elias A, Antin JH, Greenough T, Eder JP. Hepatic venoocclusive disease in autologous bone marrow transplantation of solid tumors and lymphomas. J Clin Oncol. 1990. 8:1699–1706.
Article5. Hasegawa S, Horibe K, Kawabe T, Kato K, Kojima S, Matsuyama T, Hirabayashi N. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation in children with hematologic malignancies: incidence, onset time and risk factors. Bone Marrow Transplant. 1998. 22:1191–1197.
Article6. Ozkaynak MF, Weinberg K, Kohn D, Sender L, Parkman R, Lenarsky C. Hepatic veno-occlusive disease post-bone marrow transplantation in children conditioned with busulfan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant. 1991. 7:467–474.7. Meresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L, Lemerle J. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992. 10:135–141.8. Rosenthal J, Sender L, Secola R, Killen R, Millerick M, Murphy L, Cairo MS. Phase II trial of heparin prophylaxis for veno-occlusive disease of the liver in children undergoing bone marrow transplantation. Bone Marrow Transplant. 1996. 18:185–191.9. Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995. 85:3005–3020.
Article10. Rozman C, Carreras E, Qian C, Galo RP, Bortin MM, Rowlings PA, Ash RC, Champlin RF, Henslee-Downey PJ, Herzig RH, Hinterberger W, Klein JP, Prentice HG, Reiffers J, Zwaan FE, Horowitz MM. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant. 1996. 17:75–80.11. Kami M, Mori S, Tanikawa S, Akiyama H, Onozawa Y, Tanaka T, Okamoto R, Maeda Y, Sasaki T, Kaku H, Matsuura Y, Hiruma K, Sakamaki H. Risk factors for hepatic veno-occlusive disease after bone marrow transplantation: retrospective analysis of 137 cases at a single institution. Bone Marrow Transplant. 1997. 20:397–402.
Article12. Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, Alyea EP, Antin JH, Stone RM, Soiffer RJ, DeAngelo DJ. Prior gemtuzumab ozogamicin exposure significantly increases the risk of venoocclusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003. 102:1578–1582.
Article13. Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002. 99:2310–2314.
Article14. Giles FJ, Kantarjian HM, Kornblau SM, Thomas DA, Garcia-Manero G, Waddelow TA, David CL, Phan AT, Colburn DE, Rashid A, Estey EH. Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer. 2001. 92:406–413.
Article15. Nabhan C, Rundhaugen L, Jatoi M, Riley MB, Boehlke L, Peterson LC, Tallman MS. Gemtuzumab ozogamicin (Mylotarg™) is infrequently associated with sinusoidal obstructive syndrome/veno-occlusive disease. Ann Oncol. 2004. 15:1231–1236.16. Tack DK, Letendre L, Kamath PS, Tefferi A. Development of hepatic veno-occlusive disease after Mylotarg infusion for relapsed acute myeloid leukemia. Bone Marrow Transplant. 2001. 28:895–897.
Article17. Cohen AD, Luger SM, Sickles C, Mangan PA, Porter DL, Schuster SJ, Tsai DE, Nasta S, Gewirtz AM, Stadtmauer EA. Gemtuzumab ozogamicin (Mylotarg) monotherapy for relapsed AML after hematopoietic stem cell transplant: efficacy and incidence of hepatic venoocclusive disease. Bone Marrow Transplant. 2002. 30:23–28.
Article18. Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL, Voigt J, Brousset P, Selves J, Muller C, Pris J, Laurent G. Prevention of hepatic venoocclusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992. 79:2834–2840.19. Park SH, Lee MH, Lee H, Kim K, Kim WS, Jung CW, Im YH, Yoon SS, Kang WK, Park K, Park CH, Kim SW. A randomized trial of heparin plus ursodiol vs heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002. 29:137–143.
Article20. Vaughan DE, Plavin SR, Schafer AI, Loscalzo J. PGE1 accelerates thrombolysis by tissue plasminogen activator. Blood. 1989. 73:1213–1217.21. Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H, Lehn P, Faure P, Drouet L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. 1990. 74:277–281.
Article22. Cho B, Sung IK, Kim DW, Lee HJ, Kim SY, Chung NG, Kim CC, Kim HK. A trial use of prophylactic low-dose lipo PGE1 (Eglandin) for the prevention of hepatic veno-occlusive disease after hematopoietic stem cell transplantation in children with hematologic malignancies. Korean J Pediatr Hematol Oncol. 2000. 7:242–248.23. Litzow MR, Repoussis PD, Schroeder G, Wismayer DS, Batts KP, Anderson PM, Arndt CA, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, Tefferi A, Noel P, Solberg LA Jr, Letendre L, Hoagland HC. Veno-occlusive disease of the liver after blood and marrow transplantation: analysis of pre- and post-transplant risk factors associated with severity and results of therapy with tissue plasminogen activation. Leuk Lymphoma. 2002. 43:2099–2107.24. Barker CC, Butner JD, Anderson RA, Brant R, Sauve RS. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Tranaplant. 2003. 32:79–87.
Article25. Reiss U, Cowan M, McMillian A, Horn B. Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol. 2002. 24:746–750.
Article26. Ringden O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindelev L, Parkkali T, Lenhoff S, Sallerfors B, Ljungman P, Mellander L, Jacobsen N. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994. 83:2723–2730.
Article27. Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D, Strahm B, Gross Wieltsch U, Sykora KU, Ridolfi-Lüthy A, Basu O, Gruhn B, Güngör T, Mihatsch W, Schulz AS. Defibrotide in the treatment of children with veno-occlusive disease: a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Tranaplant. 2004. 33:189–195.28. Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, Strahm B, Gross-Wieltsch U, Sykora KW, Ridolfi-Lüthy A, Basu O, Gruhn B, Güngör T, Mihatsch W, Schulz AS. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002. 100:4337–4343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic veno-occlusive disease resulting in tacrolimus toxicity after allogeneic hematopoietic stem cell transplantation
- A Trial Use of Prophylactic Low-Dose Lipo PGE1 (Eglandin) for the Prevention of Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation in Children with Hematologic Malignancies
- Hepatic Veno-occlusive Disease after Allogeneic Bone Marrow Transplantation in Patients with Leukemia: Risk Factors and Therapeutic Effect of Recombinant Human Tissue Plasminogen Activator
- Hepatic Veno-occlusive Disease Following Hematopoietic Stem Cell Transplantation in Children: Retrospective Analysis in a Single Institution
- Severe Hepatic Sinusoidal Obstruction Syndrome in a Child Receiving Vincristine, Actinomycin-D, and Cyclophosphamide for Rhabdomyosarcoma: Successful Treatment with Defibrotide