J Korean Med Sci.
2005 Oct;20(5):870-876. 10.3346/jkms.2005.20.5.870.
Effects of Hypertonic (7%) Saline on Brain Injury in Experimental Escherichia coli Meningitis
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. mhlee@smc.samsung.co.kr
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1781774
- DOI: http://doi.org/10.3346/jkms.2005.20.5.870
Abstract
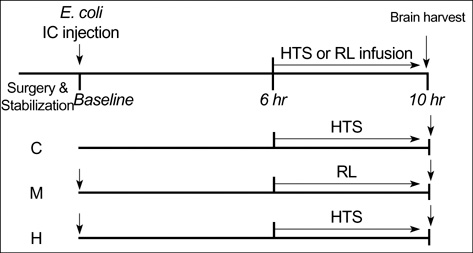
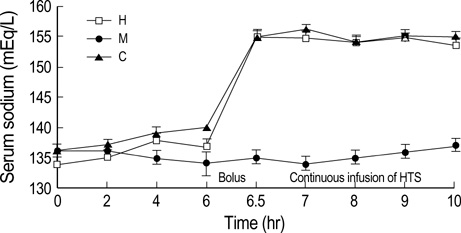
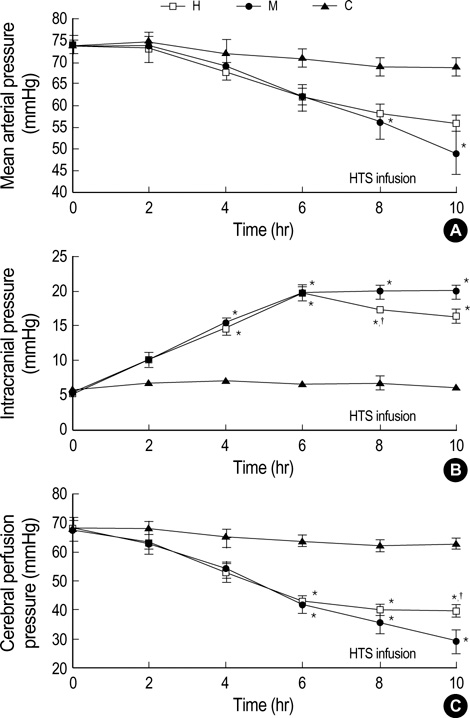
- We sought to know whether hypertonic (7%) saline (HTS) attenuates brain injury by improving cerebral perfusion pressure (CPP) and down-modulating acute inflammatory responses in experimental bacterial meningitis in the newborn piglet. Twenty-five newborn piglets were assorted into three groups: 6 in the control group (C), 10 in the meningitis group (M), and 9 in the meningitis with HTS infusion group (H). Meningitis was induced by intracisternal injection of 10(8) colony forming units of Escherichia coli in 100 microliter of saline. 10 mL/kg of HTS was given intravenously as a bolus 6 hr after induction of meningitis, thereafter the infusion rate was adjusted to maintain the serum sodium level between 150 and 160 mEq/L. HTS significantly attenuated meningitis-induced brain cell membrane disintegration and dysfunction, as indicated by increased lipid peroxidation products and decreased Na+, K+-ATPase activity in the cerebral cortex in M. HTS significantly attenuated acute inflammatory markers such as increased intracranial pressure, elevated lactate level and pleocytosis in the cerebrospinal fluid observed in M. Reduced CPP observed in M was also significantly improved with HTS infusion. These findings implicate some attenuation of the meningitis-induced alterations in cerebral cortical cell membrane structure and function with HTS, possibly by improving CPP and attenuating acute inflammatory responses.
Keyword
MeSH Terms
-
Animals
Animals, Newborn
Anti-Inflammatory Agents/administration and dosage
Brain Diseases/*drug therapy/*pathology
Cerebral Cortex/*drug effects/*pathology
Disease Models, Animal
Intracranial Pressure/drug effects
Meningitis, Escherichia coli/complications/*drug therapy/*pathology
Research Support, Non-U.S. Gov't
Saline Solution, Hypertonic/*administration and dosage
Swine
Treatment Outcome
Figure
Reference
-
1. De Louvois J. Acute bacterial meningitis in the newborn. J Antimicrob Chemother. 1994. 34:Suppl. 61–73.
Article2. Nau R, Bruck W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 2002. 25:38–45.
Article3. Park WS, Chang YS, Lee M. Effect of induced hyperglycemia on brain cell membrane function and energy metabolism during the early phase of experimental meningitis in newborn piglets. Brain Res. 1998. 798:195–203.
Article4. Park WS, Chang YS. Effects of decreased cerebral perfusion pressure on cerebral hemodynamics, brain cell membrane function and energy metabolism during the early phase of experimental Escherichia coli meningitis in the newborn piglet. J Korean Med Sci. 2000. 15:203–210.5. Tureen JH. Cerebral blood flow and metabolism in experimental meningitis. Pediatr Infect Dis J. 1989. 8:917–918.
Article6. Kreimeier U, Messmer K. Small-volume resuscitation: from experimental evidence to clinical routine. Advantages and disadvantages of hypertonic solutions. Acta Anaesthesiol Scand. 2002. 46:625–638.
Article7. Tollofsrud S, Kramer GC. Vincent JL, editor. Intra-operative use of hypertonic solutions. Yearbook of intensive care and emergency medicine. 2000. Berlin Heidelberg: Springer;476–485.8. Thomas SJ, Kramer GC, Herndon DN. Burns: military options and tactical solutions. J Trauma. 2003. 54:Suppl 5. S207–S218.9. Oliveira RP, Weingartner R, Ribas EO, Moraes RS, Friedman G. Acute haemodynamic effects of a hypertonic saline/dextran solution in stable patients with severe sepsis. Intensive Care Med. 2002. 28:1574–1581.10. Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004. 10:126–131.
Article11. Onarheim H. Fluid shifts following 7% hypertonic saline (2400 mosmol/L) infusion. Shock. 1995. 3:350–354.12. Elgjo GI, Mathew BP, Poli de Figueiredo LF, Schenarts PJ, Horton JW, Dubick MA, Kramer GC. Resuscitation with hypertonic saline dextran improves cardiac function in vivo and ex vivo after burn injury in sheep. Shock. 1998. 9:375–383.
Article13. Behrman SW, Fabian TC, Kudsk KA, Proctor KG. Microcirculatory flow changes after initial resuscitation of hemorrhagic shock with 7.5% hypertonic saline/6% dextran 70. J Trauma. 1991. 31:589–598.
Article14. Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000. 28:3301–3313.
Article15. Schmoker JD, Zhuang J, Shackford SR. Hypertonic fluid resuscitation improves cerebral oxygen delivery and reduces intracranial pressure after hemorrhagic shock. J Trauma. 1991. 31:1607–1613.
Article16. Angle N, Cabello-Passini R, Hoyt DB, Loomis WH, Shreve A, Namiki S, Junger WG. Hypertonic saline infusion: can it regulate human neutrophil function? Shock. 2000. 14:503–508.17. Pingle SC, Sanchez JF, Hallam DM, Williamson AL, Maggirwar SB, Ramkumar V. Hypertonicity inhibits lipopolysaccharide-induced nitric oxide synthase expression in smooth muscle cells by inhibiting nuclear factor kappaB. Mol Pharmacol. 2003. 63:1238–1247.18. Ciesla DJ, Moore EE, Musters RJ, Biffl WL, Silliman CA. Hypertonic saline alteration of the PMN cytoskeleton: implications for signal transduction and the cytotoxic response. J Trauma. 2001. 50:206–212.
Article19. Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000. 28:1136–1143.
Article20. Harik SI, Doull GH, Dick AP. Specific ouabain binding to brain microvessels and choroid plexus. J Cereb Blood Flow Metab. 1985. 5:156–160.
Article21. Kovachich GB, Mishra OP. Partial inactivation of Na+, K+-ATPase in cortical brain slices incubated in normal Krebs-Ringer phosphate medium at 1 and at 10 atm oxygen pressures. J Neurochem. 1981. 36:333–335.22. Recknagel RO, Glende EA Jr. Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol. 1984. 105:331–337.23. Lamprecht WA, Stein P, Heinz F. Bergmeyer HU, editor. Creatine phosphate. Methods of enzymatic analysis. 1974. New York: 1771–1781.
Article24. Powers KA, Woo J, Khadaroo RG, Papia G, Kapus A, Rotstein OD. Hypertonic resuscitation of hemorrhagic shock upregulates the antiinflammatory response by alveolar macrophages. Surgery. 2003. 134:312–318.
Article25. Park WS, Chang YS, Lee M. Effect of α-phenyl-n-tert-butylnitrone on brain cell membrane function and energy metabolism in experimental Escherichia coli meningitis in the newborn piglet. J Neurochem. 2000. 74:763–769.
Article26. Park WS, Chang YS, Shim JW, Kim MJ, Ko SY, Kim SS, Hwang JH, Choi CW, Lee M. Effects of dopamine infusion on cerebral blood flow, brain cell membrane function and energy metabolism in experimental Escherichia coli meningitis in the newborn piglet. J Korean Med Sci. 2003. 18:869–875.27. Tureen JH, Täuber MG, Sande MA. Effect of hydration status on cerebral blood flow and cerebrospinal fluid lactic acidosis in rabbits with experimental meningitis. J Clin Invest. 1992. 89:947–953.
Article28. Täuber MG, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin Infect Dis. 1999. 28:1–12.
Article29. Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986. 247:1–11.
Article30. Yeagle PL. Lipid regulation of cell membrane structure and function. FASEBJ. 1989. 3:1833–1842.31. Lees GJ. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Rev. 1991. 16:283–300.
Article32. Park WS, Chang YS, Lee M. Effect of hypothermia on brain cell membrane function and energy metabolism in experimental Escherichia coli meningitis in the newborn piglet. Neurochem Res. 2001. 26:369–374.33. Park WS, Chang YS, Lee M. 7-Nitroindazole, but not aminoguanidine, attenuates the acute inflammatory responses and brain injury during the early phase of Escherichia coli meningitis in the newborn piglet. Biol Neonate. 2001. 80:53–59.34. Park WS, Chang YS, Ko SY, Kang MJ, Han JM, Lee M. Efficacy of anti-tumor necrosis factor-alpha antibody as an adjunctive therapy in experimental Escherichia coli meningitis in the newborn piglet. Biol Neonate. 1999. 75:377–387.35. Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979. 3:79–83.
Article36. Weisman LE, Stoll BJ, Cruess DF, Hall RT, Merenstein GB, Hemming VG, Fischer GW. Early-onset group B streptococcal sepsis: a current assessment. J Pediatr. 1992. 121:428–433.
Article37. Park WS, Chang YS, Ko SY, Kang MJ, Han JM, Lee M. Effects of microbial invasion on cerebral hemodynamics and oxygenation monitored by near infrared spectroscopy in experimental Escherichia coli meningitis in the newborn piglet. Neurol Res. 1999. 21:391–398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of hypertonic saline treatment in meningitis with cerebral edema
- The Effect of Hypertonic Saline and Mannitol against Edema Formation after Cryogenic Brain Injury in Rats
- Effects of Topiramate on the Brain Cell Energy Metabolism in the Early Phase of Experimental Escherichia coli Meningitis
- A Comparison of the Cerebral and Hemodynamic Effects of Mannitol and Hypertonic Saline in a Rabbit Model of Brain Injury
- Effects of decreased cerebral perfusion pressure on cerebral hemodynamics, brain cell membrane function and energy metabolism during the early phase of experimental Escherichia coli meningitis in the newborn piglet