J Korean Med Sci.
2005 Oct;20(5):770-776. 10.3346/jkms.2005.20.5.770.
Tissue Microarray Analysis of Fas and FasL Expressions in Human Non-small Cell Lung Carcinomas; with Reference to the p53 and bcl-2 Overexpressions
- Affiliations
-
- 1Department of Pathology, Dankook University College of Medicine, Cheonan, Korea. myongnh@hanmail.net
- KMID: 1781759
- DOI: http://doi.org/10.3346/jkms.2005.20.5.770
Abstract
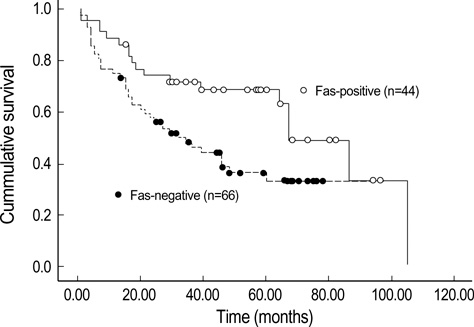
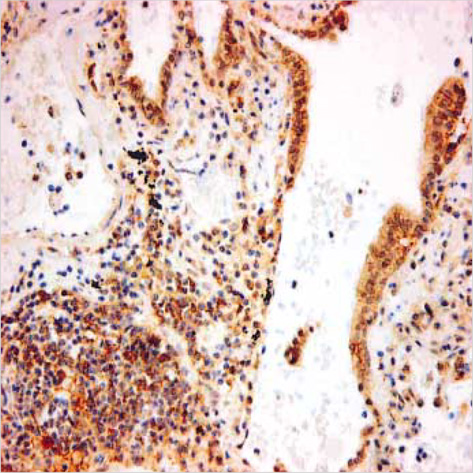
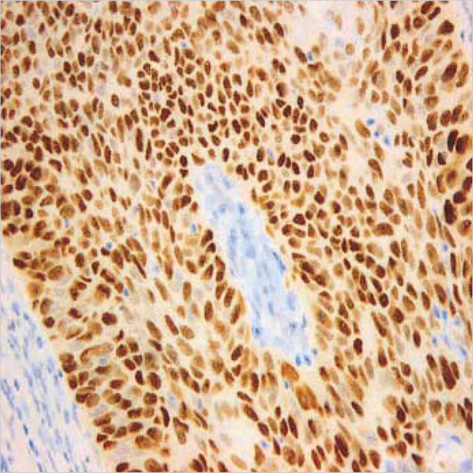
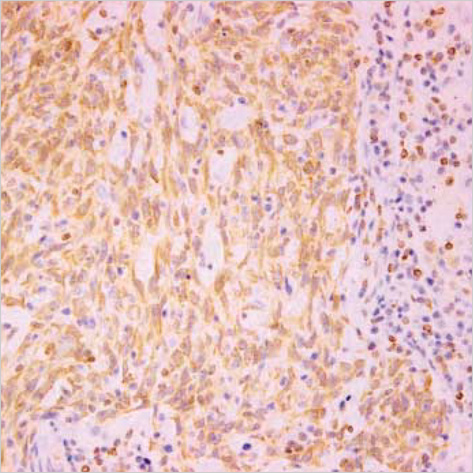
- Lack of surface Fas expression is a main route for apoptotic resistance which is considered an important mechanism of tumorigenesis and tumor progression. Fas and FasL expressions in 110 non-small cell lung carcinomas (NSCLCs) were investigated to evaluate their roles in pulmonary carcinogenesis and to examine the clinicopathologic significance of Fas expression with its relationship with p53 and bcl-2 overexpressions. Immunohistochemical analysis using tissue microarray demonstrated that a large proportion of NSCLC patients (60%) showed lack of membranous Fas expression. The Fas-negative cases revealed the significantly lower survival rate than Fas-positive ones. Also, the loss of Fas receptor expression was found more frequently in advanced stage and higher nodal status. FasL protein was increased in most NSCLCs (89%) compared to normal lungs. p53 and bcl-2 overexpressions showed no association with Fas expression. Conclusively, reduced membranous Fas expression as a mechanism of apoptotic resistance is considered to play an important part of the pulmonary carcinogenesis, which may predict poor survival and have a bad prognostic influence. Increased FasL expression is thought to be a basis for the immune evasion in NSCLCs. The rare bcl-2 overexpression suggests that this anti-apoptotic protein is unlikely to play a role in the apoptotic resistance of NSCLCs.
Keyword
MeSH Terms
-
Antigens, CD95/*metabolism
Apoptosis
Carcinoma, Non-Small-Cell Lung/*metabolism/mortality
Cell Survival
Comparative Study
Female
Gene Expression Profiling
Gene Expression Regulation, Neoplastic
Humans
Korea/epidemiology
Lung Neoplasms/*metabolism/mortality/pathology
Male
Membrane Glycoproteins/*metabolism
Middle Aged
Oligonucleotide Array Sequence Analysis
Prognosis
Proto-Oncogene Proteins c-bcl-2/*metabolism
Research Support, Non-U.S. Gov't
Risk Assessment/methods
Risk Factors
Survival Analysis
Survival Rate
Tumor Markers, Biological/*metabolism
Tumor Necrosis Factors/*metabolism
Tumor Suppressor Protein p53/*metabolism
Figure
Cited by 1 articles
-
Activated Protein C Protects Myocardium Via Activation of Anti-apoptotic Pathways of Survival in Ischemia-reperfused Rat Heart
Jia-Wang Ding, Xiao-Hong Tong, Jun Yang, Zhao-Qi Liu, Yan Zhang, Jian Yang, Song Li, Li Li
J Korean Med Sci. 2010;25(11):1609-1615. doi: 10.3346/jkms.2010.25.11.1609.
Reference
-
1. Nagata S, Goldstein P. The Fas death factor. Science. 1995. 267:1449–1456.
Article2. Wyllie AH, Kerr JF, Currie AR. Cell death: significance of apoptosis. Int Rev Cytol. 1980. 68:251–306.3. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.
Article4. Viard-Leveugle I, Veyrenc S, French LE, Brambilla C, Brambilla E. Frequent loss of Fas expression and function in human lung tumors with overexpression of FasL in small cell lung carcinoma. J Pathol. 2003. 201:268–277.5. Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA. Human lung carcinomas express Fas ligand. Cancer Res. 1997. 57:1007–1012.6. Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998. 58:1741–1749.7. O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and couterattack in the tumor-immune conflict. Ann NY Acad Sci. 2000. 910:178–192.8. Mottolese M, Buglioni S, Bracalenti C, Cardarelli MA, Ciabocco L, Giannarelli D, Botti C, Natali PG, Concetti A, Venanzi FM. Prognostic relevance of altered Fas (CD95)-system in human breast cancer. Int J Cancer. 2000. 89:127–132.
Article9. Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, Beer DG. Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest. 1998. 101:1102–1110.
Article10. Uramoto H, Osaki T, Inoue M, Taga S, Takenoyama M, Hanagiri T, Yoshino I, Nakanishi R, Ichiyoshi Y, Yasumoto K. Fas expression in non-small cell lung cancer: its prognostic effect in completely resected stage III patients. Eur J Cancer. 1999. 35:1462–1465.11. Esposito V, Baldi A, Liuzzi G, Tonini G, Vincenzi B, Persichetti P, Santini M, Ambrogi V, Mineo TC, Montesarchio V, Wolner E, Baldi F, Groeger AM. Analysis of Fas (Apo-1/CD95) expression in non-small cell lung cancer. Anticancer Res. 2003. 23:4901–4906.12. Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998. 282:290–293.
Article13. Munsch D, Watanabe-Fukunaga R, Bourdon JC, Nagata S, May E, Yonish-Rouach E, Reisdorf P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J Biol Chem. 2000. 275:3867–3872.
Article14. Fukazawa T, Fujiwara T, Morimoto Y, Shao J, Nishizaki M, Kadowaki Y, Hizuta A, Owen-Schaub LB, Roth JA, Tanaka N. Differential involvement of the CD95 (Fas/APO-1) receptor/ligand system on apoptosis induced by the wild-type p53 gene transfer in human cancer cells. Oncogene. 1999. 18:2189–2199.
Article15. Kumar V, Abbas AK, Fausto N. Cellular adaptations, cell injury, and cell death. Pathologic basis of disease. 2005. 7th ed. Philadelphia: Elsevier Saunders;3–46.16. Rodrigues NR, Rowan A, Smith MEF, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci USA. 1990. 87:7555–7559.
Article17. Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer. 1997. 80:1034–1045.18. Boldrini L, Faviana P, Pistolesi F, Gisfredi S, Quirico DD, Lucchi M, Mussi A, Angeletti CA, Baldinotti F, Fogli A, Simi P, Basolo F, Fontanini G. Alterations of Fas (APO-1/CD95) gene and its relationship with p53 in non-small cell lung cancer. Oncogene. 2001. 20:6632–6637.19. Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells-A mechanism of immune evasion? Nat Med. 1996. 2:1361–1366.20. Koomagi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999. 84:239–243.21. Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997. 94:6420–6425.
Article22. Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996. 274:1363–1366.23. Zeytun A, Nagarkatti M, Nagarkatti PS. Growth of FasL-bearing tumor cells in syngeneic murine host induces apoptosis and toxicity in Fas+ organs. Blood. 2000. 95:2111–2117.
Article24. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991. 253:49–53.
Article25. Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, Levitt M, Pass H, Gazdar AF, Minna JD. P53: a frequent target for genetic abnormalities in lung cancer. Science. 1989. 246:491–494.
Article26. Boldrini L, Faviana P, Gisfredi S, Di Quirico D, Lucchi M, Mussi A, Angeletti CA, Baldinotti F, Fogli A, Simi P, Basolo F, Pingitore F, Fontanini G. Identification of Fas (APO-1/CD95) and p53 gene mutations in non-small cell lung cancer. Int J Oncol. 2002. 20:155–159.
Article27. Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological resonsiveness. Cancer Res. 1994. 54:1580–1586.28. Itoh N, Tsuimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993. 151:621–627.29. Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001. 158:1245–1251.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of CD40 and Fas Ligand in Bowen's Disease, Squamous Cell Carcinoma and Basal Cell Carcinoma
- Differential Expressions of Fas and Fas Ligand in Human Placenta
- Expression of p63 in Lung Cancer
- Expression of bcl-2, p53 and VEGF in Non-Small Cell Lung Carcinomas: Their Relation with the Microvascular Density and Prognosis
- Immunohistochemical Study on the Expression of p53 and Bcl-2 Proteins in Non-Small Cell Lung Carcinomas