J Korean Med Sci.
2005 Oct;20(5):752-758. 10.3346/jkms.2005.20.5.752.
Expression of p63 in Reactive Hyperplasias and Malignant Lymphomas
- Affiliations
-
- 1Department of Pathology, College of Medicine, Hanyang University, Seoul, Korea. yhoh@hanyang.ac.kr
- KMID: 1781756
- DOI: http://doi.org/10.3346/jkms.2005.20.5.752
Abstract
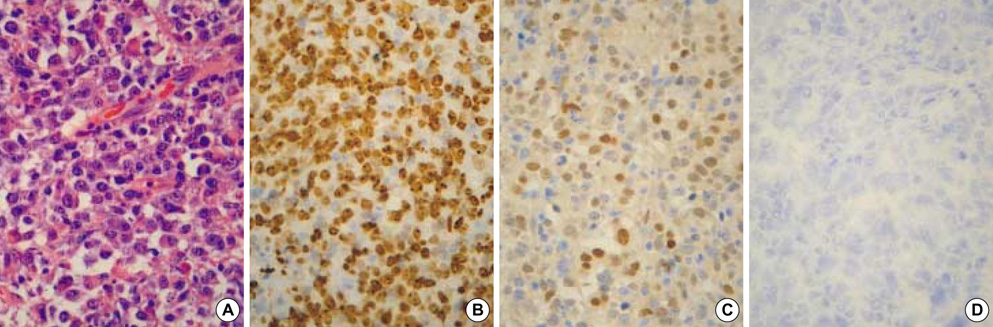
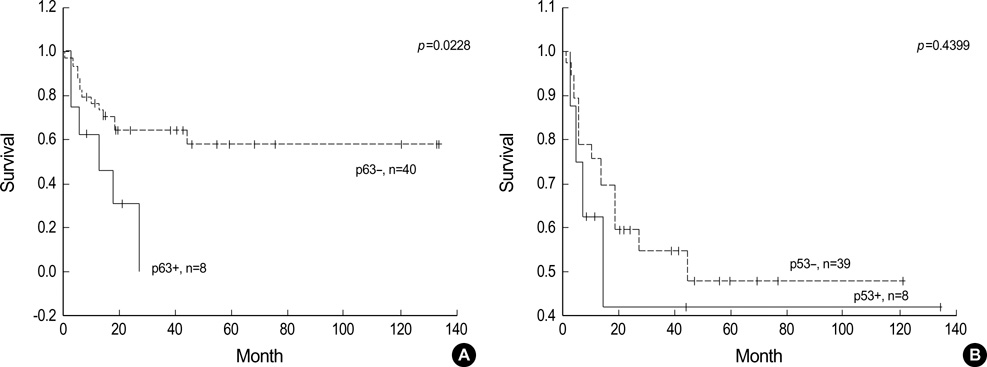
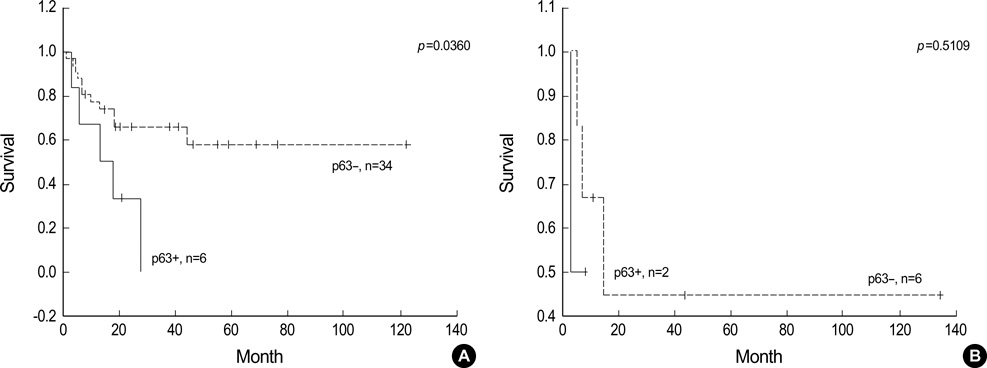
- p63 is a recently described p53 homologue. It is involved in survival and differentiation of reserve/stem cells in epithelia. To obtain new insights into the role of p63 in malignant lymphomas (MLs), immunohistochemical staining for p63 and p53 was performed in 126 cases of MLs. p63 was expressed in 38 cases of MLs (30.2%) including 32/61 cases (52.5%) of diffuse large B-cell lymphoma (DLBCL), 1/8 cases (12.5%) of precursor T-lymphoblastic lymphoma (T-LBL), 4/14 cases (28.6%) of follicular lymphoma, 1/6 cases (16.7%) of T/NK cell lymphoma. Among p63 positive cases, p63 was strongly expressed in 15/32 cases of DLBCL and 1/1 case of T-LBL. p63 was not expressed in mantle cell lymphomas, peripheral T-cell lymphomas, marginal zone B-cell lymphomas, plasma cell myelomas and Hodgkin's lymphomas. p63 was coexpressed with p53 in 18/38 p63 positive cases in which only 4 cases were strongly coexpressed. All p63+/p53+ cases were DLBCL. p63 overexpression (above 30%) cases showed significant poor survival (p=0.0228) in DLBCL. However, there was no statistically significant correlation between p63 expression and IPI score on Multivariate analysis. We could speculate that p63 could act indirectly as an oncogene by inhibiting p53 functions. Stage of differentiation of neoplastic lymphocytes appears to have a correlation with p63 expression in MLs.
MeSH Terms
Figure
Cited by 1 articles
-
Impaired Delta Np63 Expression is Associated with Poor Tumor Development in Transitional Cell Carcinoma of the Bladder
Yunfeng He, Xiaohou Wu, Wei Tang, Daiyin Tian, Chunli Luo, Zhikang Yin, Hu Du
J Korean Med Sci. 2008;23(5):825-832. doi: 10.3346/jkms.2008.23.5.825.
Reference
-
1. Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, Mckeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998. 2:305–316.
Article2. Little NA, Jochemsen AG. p63. Int J Biochem Cell Biol. 2002. 34:6–9.
Article3. Chen X. The p53 family: Same response, different signals? Mol Med Today. 1999. 5:387–392.
Article4. Shimada A, Kato S, Enjo K, Osada M, Ikawa Y, Kohno K, Obinata M, Kanamaru R, Ikawa S, Ishioka C. The transcriptional activities of p53 and its homologue p51/p63: similarities and differences. Cancer Res. 1999. 59:2781–2786.5. Yang A, Mckeon F. P63 and p73: p53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000. 1:199–207.
Article6. Urist MJ, Di Como CJ, Lu ML, Charytonowics E, Verbel D, Crum CP, Ince TA, Mckeon FD, Cordon-Cardo C. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002. 161:1199–1206.
Article7. Crook T, Nicholls JM, Brooks L, O'Nions J, Allday MJ. High level expression of deltaN-p63: a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC). Oncogene. 2000. 19:3439–3444.8. Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000. 97:5462–5467.
Article9. Hibi K, Nakayama H, Taguchi M, Kasai Y, Ito K, Akiyama S, Nakao A. AIS overexpression in advanced esophageal cancer. Clin Cancer Res. 2001. 7:469–472.10. Chilosi M, Doglioni C. Constitutive p63 expression in airway basal cells. A molecular target in diffuse lung diseases. Sarcoidosis Vasc Diffuse Lung Dis. 2001. 18:23–26.11. Glickman JN, Yang A, Shahsafaei A, Mckeon F, Odze RD. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol. 2001. 32:1157–1165.
Article12. Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001. 98:3156–3161.
Article13. De Laurenzi V, Rossi A, Terrinoni A, Barcaroli D, Levrero M, Costanzo A, Knight RA, Guerrieri P, Melino G. p63 and p73 transactivate differentiation gene promoters in human keratinocytes. Biochem Biophys Res Commun. 2000. 273:342–346.
Article14. Djelloul S, Tarunina M, Barnouin K, Mackay A, Jat PS. Differential protein expression, DNA binding and interaction with SV40 large tumor antigen implicate the p63-family of proteins in replicative senescence. Oncogene. 2002. 21:981–989.15. Senoo M, Matsumura Y, Habu S. TAp63 gamma (p51A) and ΔNp63-alpha (p73L), two major isoforms of the p63 gene, exert opposite effects on the vascular endothelial growth factor (VEGF) gene expression. Oncogene. 2002. 21:2455–2465.16. Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, Mckeon F. p63 is essential for regenerative proliferation in limb, craniofacial, and epithelial development. Nature. 1999. 398:714–718.
Article17. Levin AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature. 1991. 351:453–456.18. Ahn MJ, Kim H, Kim IS, Park JK, Ki MR, Park CK. P53 protein expression and its prognostic importance in patients with nodal non-Hodgkin's lymphoma. J Korean Med Sci. 2000. 15:59–64.
Article19. Park CK, Lee JD. Immunohistochemical and SSCP analysis of p53 in malignant lymphomas. J Korean Med Sci. 1998. 13:361–368.
Article20. Hall PA, Campbell SJ, O'Neill M, Royston DJ, Nylander K, Carey FA, Kernohan NM. Expression of the p53 homologue p63alpha and deltaNp63alpha in normal and neoplastic cells. Carcinogenesis. 2000. 21:153–160.21. Park BJ, Lee SJ, Kim JI, Lee CH, Chang SG, Park JH, Chi SG. Frequent alteration of p63 expression in human primary bladder carcinomas. Cancer Res. 2000. 60:3370–3374.22. Koga F, Kawakami S, Kumagai J, Takizawa T, Ando N, Arai G, Kageyama T, Kihara L. Impaired Delta Np63 expression associates with reduced beta catenin and aggressive phenotypes of urothelial neoplasms. Br J Cancer. 2003. 88:740–747.23. Jaffe ES, Harris NL, Stein H, Vardiman JW. Tumors of hematopoietic and lymphoid tissues, Pathology & Genetics, World Health Organization Classification of Tumors. 2001. Lyon IARC Press;127–130.24. Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002. 8:494–501.25. Chilosi M, Zamo A, Brighenti A, Malpeli G, Montagna L, Piccoli P, Pedron S, Lestani M, Inghirami G, Scarpa A, Doglioni C, Menestrina F. Constitutive expression of ΔNp63α isoform in human thymus and thymic epithelial tumors. Virchows Arch. 2003. 443:175–183.26. Frick H, Munger DM, Fauchere JC, Stallmach T. Hypoplastic thymus and T-cell reduction in EECUT syndrome. Am J Med Genet. 1997. 69:65–68.
Article27. Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999. 398:708–713.
Article28. Ogutcen-Toller M, Gulen O, Okten G, Elbistan M. Non-Hodgkin's lymphoma in a patient with ectrodactyly ectodermal dysplasia-clefting syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000. 90:124–125.29. Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, Newbury-Ecob R, Hennekam RC, Van Buggenhout G, van Haeringen A, Woods CG, van Essen AJ, de Waal R, Vriend G, Haber DA, Yang A, McKeon F, Brunner HG, van Bokhoven H. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999. 99:143–153.
Article30. Ianakiev P, Kilpatrick MW, Toudjarska I, Basel D, Beighton P, Tsipouras P. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet. 2000. 67:59–66.
Article31. McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR, Wessagowit V, Kelly A, Atherton DJ, Griffiths WA, Orlow SJ, van Haeringen A, Ausems MG, Yang A, McKeon F, Bamshad MA, Brunner HG, Hamel BC, van Bokhoven H. Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet. 2001. 10:221–229.
Article32. Wessagowit V, Mellerio JE, Pembroke AC, McGrath JA. Heterozygous germline missense mutation in the p63 gene underlying EEC syndrome. Clin Exp Dermatol. 2000. 25:441–443.
Article33. Parsa R, Yang A, Mckeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999. 113:1099–1105.
Article34. Yamaguchi K, Wu L, Caballero OL, Hibi K, Trink B, Resto V, Cairns P, Okami K, Koon WM, Sidransky D, Jen J. Frequent gain of the p40/p51/p63 gene locus in primary head and neck squamous cell carcinoma. Int J Cancer. 2000. 86:684–689.35. Ribeiro-Silva A, Zambelli Ramalho LN, Britto Garcia S, Zucoloto S. The relationship between p63 and p53 expression in normal and neoplastic breast tissue. Arch Pathol Lab Med. 2003. 127:336–340.
Article36. Zigeuner R, Tsybrovskyy O, Ratschek M, Rehak P, Lipsky K, Langner C. Prognostic impact of p63 and p53 expression in upper urinary tract transitional cell carcinoma. Urology. 2004. 63:1079–1083.
Article37. Ikawa S, Nakagawara A, Ikawa Y. p53 family genes: structural comparison, expression, and mutation. Cell Death Differ. 1999. 6:1154–1161.
Article38. Roth J, Dobbelstein M. Failure of viral oncoproteins to target the p53-homologue p51A. J Gen Virol. 1999. 80:3251–3255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of MYC gene amplification in malignant lymphomas
- Expression Patterns of Gli-1, Pleckstrin Homology-Like Domain, Family A, Member 1, Transforming Growth Factor-beta1/beta2, and p63 in Sebaceous and Follicular Tumors
- The Clinicopathologic Significance of a p63 Expression in Invasive Breast Cancer
- Immunoglobulin and T-cell receptor gene rearrangement analysis in malignant lymphoid neoplasms
- Expression of p63,bcl-2,bcl-6 and p16 in Basal Cell Carcinoma and Squamous Cell Carcinoma of the Skin