J Korean Med Sci.
2005 Feb;20(1):132-138. 10.3346/jkms.2005.20.1.132.
Effects of Methylprednisolone on the Neural Conduction of the Motor Evoked Potentials in Spinal Cord Injured Rats
- Affiliations
-
- 1Department of Neurosurgery, Medical Research Center, Brain Research Institute, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. electro@yumc.yonsei.ac.kr
- 2Department of Neurosurgery, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- KMID: 1781740
- DOI: http://doi.org/10.3346/jkms.2005.20.1.132
Abstract
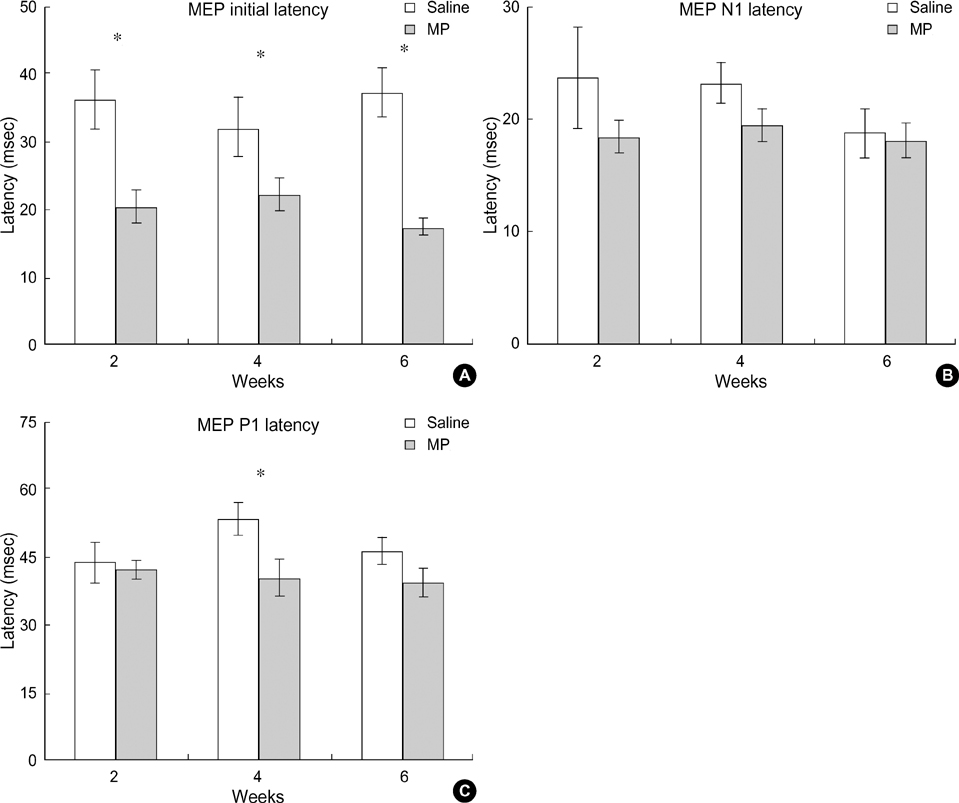
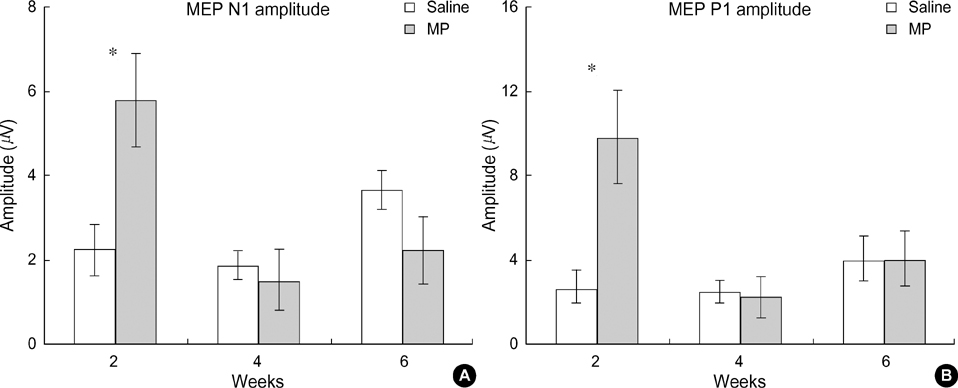
- Methylprednisolone (MP), a glucocorticoid steroid, has an anti-inflammatory action and seems to inhibit the formation of oxygen free radicals produced during lipid peroxidation in a spinal cord injury (SCI). However, the effects of MP on the functional recovery after a SCI is controversial. The present study was conducted to determine the effects of MP on the recovery of neural conduction following a SCI. A SCI was produced using the NYU spinal cord impactor. A behavioral test was conducted to measure neurological disorders, and motor evoked potentials (MEPs) were recorded. According to the behavioral test, using BBB locomotor scaling, MP-treated animals showed improved functional recoveries when compared to salinetreated animals. MEP latencies in the MP-treated group were shortened when compared to those in the control group. Peak amplitudes of MEPs were larger in the MP-treated group than those in the control group. The thresholds of MEPs tended to be lower in the MP-treated group than those in the control group. These results suggest that MP may improve functional recovery after a SCI.
MeSH Terms
-
Animals
Disease Models, Animal
Electrophysiology
Evoked Potentials, Motor/*drug effects
Free Radicals
Glucocorticoids/metabolism
Male
Methylprednisolone/*pharmacology
Neurons/*drug effects
Oxygen/metabolism
Rats
Rats, Sprague-Dawley
Receptors, Glucocorticoid/metabolism
Research Support, Non-U.S. Gov't
Sodium Chloride/pharmacology
Spinal Cord/pathology
Spinal Cord Injuries/*drug therapy
Time Factors
Figure
Cited by 1 articles
-
Evaluation of the Combination of Methylprednisolone and Tranilast after Spinal Cord Injury in Rat Models
Ngwayi James Reeves Mbori, Xie Yun Chuan, Qiao Xiao Feng, Mujahid Alizada, Jing Zhan
J Korean Neurosurg Soc. 2016;59(4):334-340. doi: 10.3340/jkns.2016.59.4.334.
Reference
-
1. Collins WF, Piepmeir J, Ogle E. The spinal cord injury problem: A review. Central Nerv Syst Trauma. 1986. 3:317–331.2. Anderson TE, Stokes BT. Experimental models for spinal cord injury research: Physical and Physiological considerations. J Neurotrauma. 1992. 9:S135–S142.3. O'Brien MF, Lenke LG, Lou J, Bridwell KH, Joyce ME. Astrocyte response and transforming growth factor-β localization in acute spinal cord injury. Spine. 1994. 19:2321–2330.4. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991. 75:15–26.
Article5. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, Marshall LF, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner FC, Wilberger JE, Winn HR. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990. 322:1405–1411.6. Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992. 76:13–22.
Article7. Ross IB, Tator CH. Spinal cord blood flow and evoked potential responses after treatment with nimodipine or methylprednisolone in spinal cord injured rats. Neurosurgery. 1993. 33:470–477.8. Ross IB, Tator CH, Theriault E. Effect of nimodipine or methylprednisolone on recovery from acute experimental spinal cord injury in rats. Surg Neurol. 1993. 40:461–470.
Article9. Koyanagi I, Tator CH. Effect of a single huge dose of methylprednisolone on blood flow, evoked potentials, and histology after acute spinal cord injury in the rat. Neurol Res. 1997. 19:289–299.
Article10. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.
Article11. Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990. 13:43–60.12. Raivich G, Kreutzberg GW. Peripheral nerve regeneration: role of growth factors and their receptors. Int J Dev Neurosci. 1993. 11:311–324.
Article13. Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996. 76:319–370.
Article14. Nicholls JG, Adams WB, Eugenin J, Geiser R, Lepre M, Luque JM, Wintzer M. Why does the central nervous system not regenerate after injury? Surv Ophthalmol. 1999. 43:S136–S141.
Article15. Holtz A, Nystrom B, Gerdin B. Effect of methylprednisolone of motor function and spinal cord blood flow after spinal cord compression in rats. Acta Neurol Scand. 1990. 82:68–73.16. Campbell JB, DeCrescito V, Tomasula JJ, Demopoulos HB, Flamm ES, Ransohoff J. Experimental treatment of spinal cord contusion in the cat. Surg Neurol. 1973. 1:102–106.17. Braughler JM, Hall EX, Means ED, Waters TR, Anderson DK. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal cord injury. J Neurogurg. 1987. 67:102–105.
Article18. Green BA, Kahn T, Klose KJ. A comparative study of steroid therapy in acute experimental spinal cord injury. Surg Neurol. 1980. 13:91–97.19. Hedeman LS, Sil R. Studies in experimental spinal cord trauma. Part 2: Comparison of treatment with steroids, low molecular weight dextran, and catecholamine blockade. J Neurosurg. 1974. 40:44–51.20. Faden AI, Jacobs TP, Patrick DH, Smith MT. Megadose corticosteroid therapy following experimental traumatic spinal injury. J Neurosurg. 1984. 60:712–717.
Article21. Iizuka H, Iwasaki Y, Yamamoto T, Kadoya S. Morphometric assessment of drug effects in experimental spinal cord injury. J Neurosurg. 1986. 65:92–98.
Article22. Kelly DF, Bernstein JJ, Laws ER. Methylprednisolone: Therapeutic time window in experimental spinal cord injury in rats. Surg Forum. 1991. 42:512–514.23. Iwai A, Monafo WW, Elliasson SG. Methylprednisolone treatment of exerimental spinal cord injury. Paraplegia. 1993. 31:417–429.24. Young W, Flamm ES. Effect of high-dose corticosteroid therapy on blood flow, evoked potentials, and extracellular calcium in experimental spinal injury. J Neurosurg. 1982. 57:667–673.
Article25. De Ley G, Leybaert L. Effect of flunarizine and methylprednisolone on functional recovery after experimental spinal injury. J Neurotrauma. 1993. 10:25–35.
Article26. Hsu CY, Dimitrijevic MR. Methylprednisolone in spinal cord injury: the possible mechanism of action. J Neurotrauma. 1990. 7:115–119.
Article27. Hirata F, Schiffmann E, Venkatasubramanian K, Salomon D, Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci USA. 1980. 77:2533–2536.
Article28. Becker J, Grasso RJ. Suppression of phagocytosis by dexamethasone in macrophage cultures: inability of arachidonic acid, indomethacin, and nordihydroguaiaretic acid to reverse the inhibitory response mediated by a steroid-inducible factor. Int J Immunopharmacol. 1985. 7:839–847.
Article29. Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Mol Brain Res. 1998. 59:135–142.30. Young W, DeCrescito V, Flamm ES, Blight AR, Gruner JA. Pharmacological therapy of acute spinal cord injury: studies of high dose methylprednisolone and naloxone. Clin Neurosurg. 1988. 34:675–697.31. Hall ED, Wolf DL, Braughler JM. Effects of a single large dose of methylprednisolone sodium succinate on experimental posttraumatic spinal cord ischemia. Dose-response and time-action analysis. J Neurosurg. 1984. 61:124–130.32. Demopoulos HB, Flamm ES, Pietronigro DD, Seligman ML. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980. 492:91–119.33. Nash HH, Borke RC, Anders JJ. Ensheathing cells and methylprednisolone promote axonal regeneration and functional recovery in the lesioned adult rat spinal cord. J Neurosci. 2002. 22:7111–7120.
Article34. Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000. 17:203–218.
Article35. Kim DH, Jahng TA. Continuous brain-derived neurotrophic factor (BDNF) infusion after methylprednisolone treatment in severe spinal cord injury. J Korean Med Sci. 2004. 19:113–122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of motor evoked potentials and spinal cord evoked potentials following spinal cord injury in rats
- Usefulness of motor evoked potentials in the spinal cord injured rat
- Failure of topical DMSO to improve blood flow or evoked potentials in rat spinal cord injury
- Intraoperative Monitoring for Tethered Cord Syndrome Using Somatosensory Evoked Potential and Motor Evoked Potential: Report of three cases
- Motor Evoked Potentials and Recovery of Motor Function in Spinal Cord Injuried Rats