Korean J Lab Med.
2007 Apr;27(2):89-95. 10.3343/kjlm.2007.27.2.89.
Relationship between In Vitro Chemosensitivity assessed with MTT Assay and Clinical Outcomes in 103 Patients with Acute Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cjpark@amc.seoul.kr
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1781465
- DOI: http://doi.org/10.3343/kjlm.2007.27.2.89
Abstract
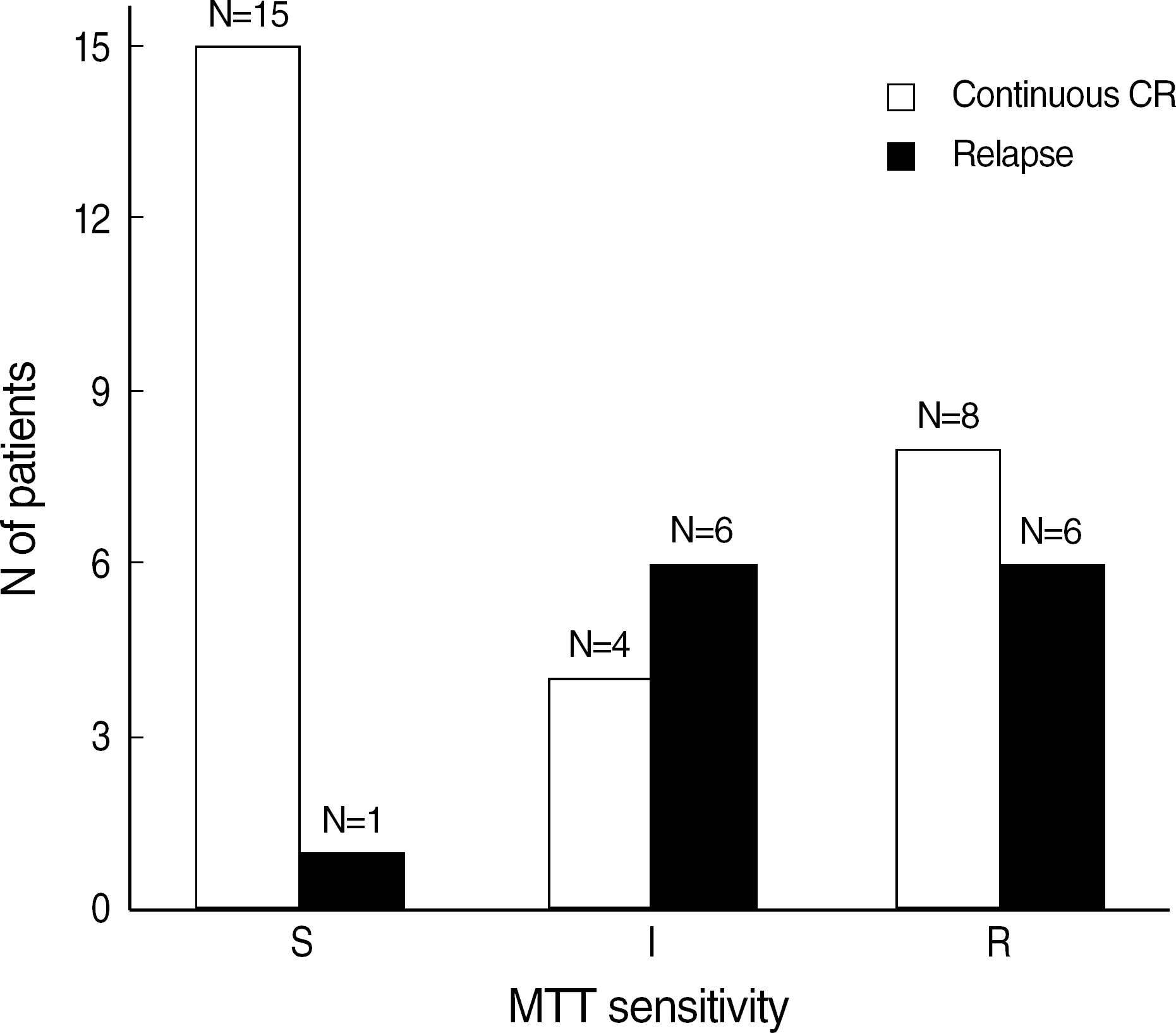
- BACKGROUND: Cellular drug resistance is supposed to play a major role in chemotherapy failure or relapse. The purpose of this study was to analyze the relationship between in vitro chemosensitivity test results using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and clinical response on chemotherapy, and to find the possibility of optimizing the treatment protocol for individual patients according to their actual drug resistance. METHODS: For MTT assay, we obtained bone marrow aspirates from 103 patients with acute leukemia at the time of initial diagnosis or relapse. The following drugs were tested: cytarabine, vincristine, methotrexate, daunorubicin, dexamethasone, L-asparaginase, and mitoxantrone. To evaluate clinical responses after induction chemotherapy, we followed up on their bone marrow study. RESULTS: In our study, in vitro chemosensitivity test with the MTT assay significantly predicted whether patients with AML remained continuous complete remission or went into relapse. It also predicted whether or not child patients with ALL would acquire complete remission after induction chemotherapy. CONCLUSIONS: Although it does not provide the insight into the mechanisms that cause drug resistance, the MTT assay may be a useful tool in individually optimizing the chemotherapy of patients with acute leukemia.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Antibiotics, Antineoplastic/therapeutic use
Child
Child, Preschool
Coloring Agents
Cytarabine/therapeutic use
Daunorubicin/therapeutic use
Drug Evaluation, Preclinical
Drug Resistance, Neoplasm
Female
Humans
Infant
Leukemia, Biphenotypic, Acute/diagnosis/*drug therapy
Leukemia, Myeloid, Acute/diagnosis/*drug therapy
Male
Middle Aged
Precursor Cell Lymphoblastic Leukemia-Lymphoma/diagnosis/*drug therapy
Tetrazolium Salts
Thiazoles
Treatment Outcome
Figure
Reference
-
References
1. Creutzig U, Ritter J, Zimmermann M, Hermann J, Gadner H, Sawatzki DB, et al. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML-BFM 93. AML-BFM Study Group. Leukemia. 2001; 15:348–54.2. Veerman AJ, Pieters R. Drug sensitivity assays in leukaemia and lymphoma. Br J Haematol. 1990; 74:381–4.
Article3. Kim HK, Kim YK, Kim DW, Paik SY. Study on the Optimal Storage Condition for Granulocyte Transfusion using MTT assay. Korean J Clin Pathol. 1990; 10:461–9.4. Morabito F, Stelitano C, Callea I, Dattola A, Console G, Pucci G, et al. In vitro drug-induced cytotoxicity predicts clinical response to flu-darabine in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1998; 102:528–31.
Article5. Styczynski J, Wysocki M. Ex vivo drug resistance in childhood acute myeloid leukemia on relapse is not higher than at first diagnosis. Pediatr Blood Cancer. 2004; 42:195–9.
Article6. Klumper E, Pieters R, Veerman AJ, Huismans DR, Loonen AH, Hahlen K, et al. In vitro cellular drug resistance in children with relapsed/refractory acute lymphoblastic leukemia. Blood. 1995; 86:3861–8.
Article7. Klumper E, Pieters R, Kaspers GJ, Huismans DR, Loonen AH, Rottier MM, et al. In vitro chemosensitivity assessed with the MTT assay in childhood acute non-lymphoblastic leukemia. Leukemia. 1995; 9:1864–9.8. Styczynski J, Pieters R, Huismans DR, Schuurhuis GJ, Wysocki M, Veerman AJ. In vitro drug resistance profiles of adult versus childhood acute lymphoblastic leukaemia. Br J Haematol. 2000; 110:813–8.
Article9. Zwaan CM, Kaspers GJ, Pieters R, Hahlen K, Janka-Schaub GE, van Zantwijk CH, et al. Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood. 2002; 99:245–51.
Article10. Garand R, Robillard N. Immunophenotypic characterization of acute leukemias and chronic lymphoproliferative disorders: practical recommendations and classifications. Hematol Cell Ther. 1996; 38:471–86.
Article11. Craddock C, Tauro S, Moss P, Grimwade D. Biology and management of relapsed acute myeloid leukaemia. Br J Haematol. 2005; 129:18–34.
Article12. Hongo T, Yajima S, Sakurai M, Horikoshi Y, Hanada R. In vitro drug sensitivity testing can predict induction failure and early relapse of childhood acute lymphoblastic leukemia. Blood. 1997; 89:2959–65.
Article13. Mollgard L, Tidefelt U, Sundman-Engberg B, Lofgren C, Paul C. In vitro chemosensitivity testing in acute non lymphocytic leukemia using the bioluminescence ATP assay. Leuk Res. 2000; 24:445–52.14. Zwaan CM, Kaspers GJ, Pieters R, Hahlen K, Huismans DR, Zimmermann M, et al. Cellular drug resistance in childhood acute myeloid leukemia is related to chromosomal abnormalities. Blood. 2002; 100:3352–60.
Article15. Guerrasio A, Pilatrino C, De Micheli D, Cilloni D, Serra A, Gottardi E, et al. Assessment of minimal residual disease (MRD) in CBFbeta/MYH11-positive acute myeloid leukemias by qualitative and quantitative RT-PCR amplification of fusion transcripts. Leukemia. 2002; 16:1176–81.
Article16. Mihal V, Hajduch M, Noskova V, Janostakova A, Safarova M, Orel M, et al. The analysis of correlations between drug resistance and clinical/laboratory measures found in a group of children with all treated by ALL-BFM 90 protocol. Bull Cancer. 2004; 91:80–9.17. Mihal V, Hajduch M, Noskova V, Feketova G, Jess K, Gojova L, et al. Differential antileukemic activity of prednisolone and dexamethasone in freshly isolated leukemic cells. Adv Exp Med Biol. 1999; 457:461–71.18. Pieters R, den Boer ML, Durian M, Janka G, Schmiegelow K, Kaspers GJ, et al. Relation between age, immunophenotype and in vitro drug resistance in 395 children with acute lymphoblastic leukemia–implications for treatment of infants. Leukemia. 1998; 12:1344–8.
Article19. Kaspers GJ, Pieters R, Van Zantwijk CH, Van Wering ER, Van Der Does-Van Den Berg A, Veerman AJ. Prednisolone resistance in childhood acute lymphoblastic leukemia: vitro-vivo correlations and cross-resistance to other drugs. Blood. 1998; 92:259–66.
Article20. Styczynski J, Wysocki M, Debski R, Juraszewska E, Malinowska I, Stanczak E, et al. Ex vivo drug resistance profile in childhood acute myelogenous leukemia: no drug is more effective in comparison to acute lymphoblastic leukemia. Leuk Lymphoma. 2002; 43:1843–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MTT and Neutral red Biologic Assay for Human Bladder Cancer Cell Line(TCC-SuP) and Androgen Stimulated Cell Line of Mouse (SC-115): In Vitro Chemosensitivity Test
- Chemosensitivity Test of Childhood Leukemic Cells
- Colony Formation Assay and Chemosensitivity Test for Urologic Malignancies: A Preliminary Report
- Comparison of anticancer drug efficacy using the short-term microplate culture and MTT dye reduction assay
- A study on chemosensitivity test in relation with the phases of cell cycle in ovarian cancer cell line