Yonsei Med J.
2014 Jan;55(1):99-106. 10.3349/ymj.2014.55.1.99.
Rosiglitazone, a Peroxisome Proliferator-Activated Receptor-gamma Agonist, Restores Alveolar and Pulmonary Vascular Development in a Rat Model of Bronchopulmonary Dysplasia
- Affiliations
-
- 1Department of Pediatrics, Hanyang University Seoul Hospital, Seoul, Korea.
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. choicw@snu.ac.kr
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1779892
- DOI: http://doi.org/10.3349/ymj.2014.55.1.99
Abstract
- PURPOSE
We tested whether rosiglitazone (RGZ), a peroxisome proliferator-activated receptor-gamma agonist, can restore alveolar development and vascular growth in a rat model of bronchopulmonary dysplasia (BPD).
MATERIALS AND METHODS
A rat model of BPD was induced through intra-amniotic delivery of lipopolysaccharide (LPS) and postnatal hyperoxia (80% for 7 days). RGZ (3 mg/kg/d, i.p.) or vehicle was given daily to rat pups for 14 days. This model included four experimental groups: No BPD+vehicle (V), No BPD+RGZ, BPD+V, and BPD+RGZ. On D14, alveolarization, lung vascular density, and right ventricular hypertrophy (RVH) were evaluated.
RESULTS
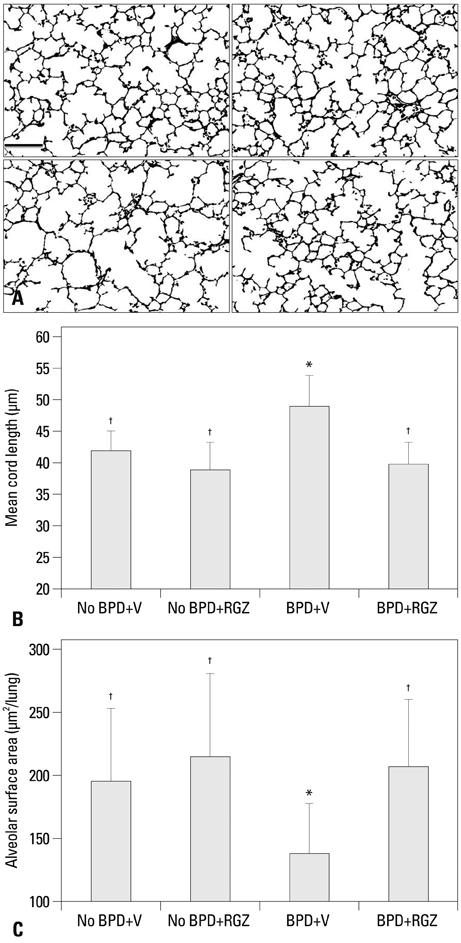
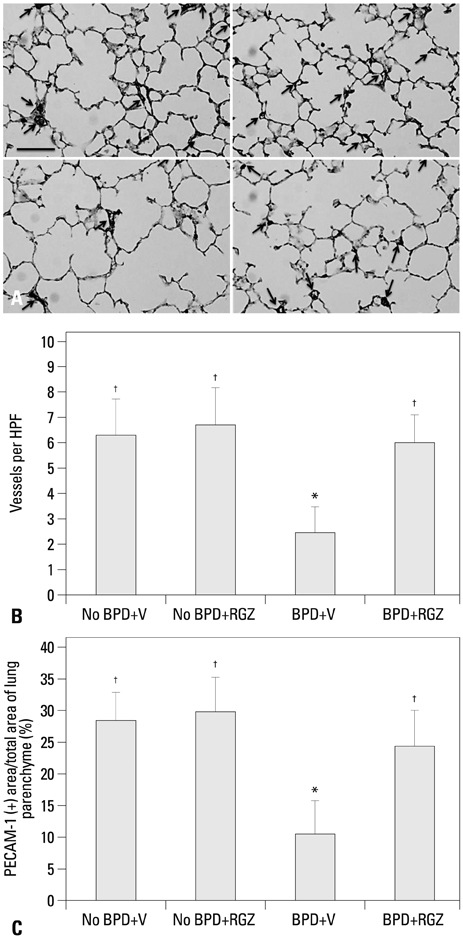
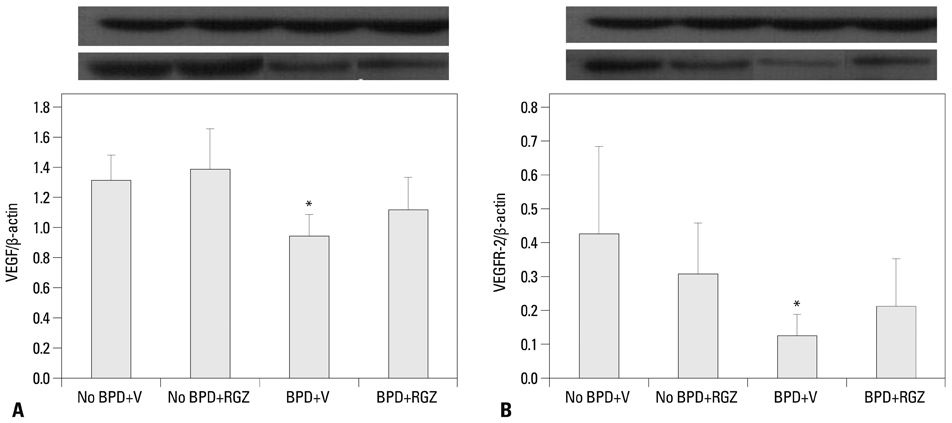
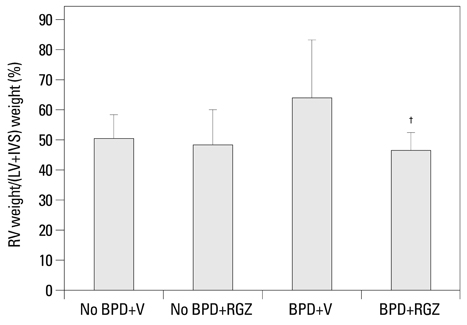
Morphometric analysis revealed that the BPD+RGZ group had significantly smaller and more complex airspaces and larger alveolar surface area than the BPD+V group. The BPD+RGZ group had significantly greater pulmonary vascular density than the BPD+V group. Western blot analysis revealed that significantly decreased levels of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 by the combined exposure to intra-amniotic LPS and postnatal hyperoxia were restored by the RGZ treatment. RVH was significantly lesser in the BPD+RGZ group than in the BPD+V group.
CONCLUSION
These results suggest that RGZ can restore alveolar and pulmonary vascular development and lessen pulmonary hypertension in a rat model of BPD.
Keyword
MeSH Terms
Figure
Reference
-
1. Philip AG. Bronchopulmonary dysplasia: then and now. Neonatology. 2012; 102:1–8.
Article2. Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F8–F17.
Article3. Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med. 2010; 38:571–577.
Article4. Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006; 11:354–362.
Article5. Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L608–L611.
Article6. McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997; 59:43–62.
Article7. Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol. 2006; 41:558–569.
Article8. Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L1031–L1041.9. Wang Y, Santos J, Sakurai R, Shin E, Cerny L, Torday JS, et al. Peroxisome proliferator-activated receptor gamma agonists enhance lung maturation in a neonatal rat model. Pediatr Res. 2009; 65:150–155.
Article10. Lee HJ, Kim BI, Choi ES, Choi CW, Kim EK, Kim HS, et al. Effects of postnatal dexamethasone or hydrocortisone in a rat model of antenatal lipopolysaccharide and neonatal hyperoxia exposure. J Korean Med Sci. 2012; 27:395–401.
Article11. Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res. 2009; 65:323–327.
Article12. Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952; 14:413–420.
Article13. Lee HJ, Choi CW, Kim BI, Kim EK, Kim HS, Choi JH, et al. Serial changes of lung morphology and biochemical profiles in a rat model of bronchopulmonary dysplasia induced by intra-amniotic lipopolysaccharide and postnatal hyperoxia. J Perinat Med. 2010; 38:675–681.
Article14. Kim DH, Choi CW, Kim EK, Kim HS, Kim BI, Choi JH, et al. Association of increased pulmonary interleukin-6 with the priming effect of intra-amniotic lipopolysaccharide on hyperoxic lung injury in a rat model of bronchopulmonary dysplasia. Neonatology. 2010; 98:23–32.
Article15. Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005; 67:623–661.
Article16. Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003; 285:L161–L168.17. Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006; 291:L1068–L1078.
Article18. Park HS, Park JW, Kim HJ, Choi CW, Lee HJ, Kim BI, et al. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am J Respir Cell Mol Biol. 2013; 48:105–113.
Article19. Biscetti F, Straface G, Pitocco D, Zaccardi F, Ghirlanda G, Flex A. Peroxisome proliferator-activated receptors and angiogenesis. Nutr Metab Cardiovasc Dis. 2009; 19:751–759.
Article20. Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L811–L823.21. Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005; 112:2477–2486.
Article22. Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 164(10 Pt 1):1971–1980.
Article23. Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007; 175:978–985.24. Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999; 354:141–148.
Article25. Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, et al. Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000; 271:571–574.
Article26. Rehan VK, Torday JS. PPARγ Signaling Mediates the Evolution, Development, Homeostasis, and Repair of the Lung. PPAR Res. 2012; 2012:289867.27. Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med. 2003; 22:189–207.
Article28. Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L130–L135.29. Rehan VK, Fong J, Lee R, Sakurai R, Wang ZM, Dahl MJ, et al. Mechanism of reduced lung injury by high-frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr Res. 2011; 70:462–466.
Article30. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998; 391:79–82.
Article31. Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu Rev Pharmacol Toxicol. 2000; 40:491–518.
Article32. Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000; 448:121–138.
Article33. Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol. 2004; 483:79–93.
Article34. Rehan VK, Dargan-Batra SK, Wang Y, Cerny L, Sakurai R, Santos J, et al. A paradoxical temporal response of the PTHrP/PPAR-gamma signaling pathway to lipopolysaccharide in an in vitro model of the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L182–L190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Sulfonylureas on Peroxisome Proliferator-Activated Receptor gamma Activity and on Glucose Uptake by Thiazolidinediones
- The Effect of Chlamydia pneumoniae on the Expression of Peroxisome Proliferator-Activated Receptor-gamma in Vascular Smooth Muscle Cells
- Differential Expression of RANKL and OPG by the PPAR gamma Agonist Rosiglitazone in Osteoblasts
- Effect of Rosiglitazone on Myocardial Ischemia-Reperfusion Injury in Rat Heart
- Expression of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma in the lung tissue of obese mice and the effect of rosiglitazone on proinflammatory cytokine expressions in the lung tissue