Treatment of Severe Atopic Dermatitis with a Combination of Subcutaneous Allergen Immunotherapy and Cyclosporin
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. donghonahm@yahoo.co.kr
- KMID: 1779701
- DOI: http://doi.org/10.3349/ymj.2012.53.1.158
Abstract
- PURPOSE
The clinical efficacy of subcutaneous allergen immunotherapy (SCIT) for the treatment of patients with severe atopic dermatitis (AD) using house dust mite (HDM) extract has been reported. Cyclosporin has been regarded as an effective medication for treatment of severe AD. In this study, we investigated a clinical usefulness of combined treatment with SCIT and cyclosporin in patients with severe AD.
MATERIALS AND METHODS
Nine patients with severe AD and hypersensitivity to HDM were treated with a combination of SCIT using HDM extract and cyclosporin for 12 months. The primary efficacy outcome was the change in the standardized clinical severity scoring system for AD (SCORAD) values, measured at 6 and 12 months, in comparison with the values at baseline. Daily dose of cyclosporin was decreased or discontinued according to the degrees of clinical improvements in individual patients.
RESULTS
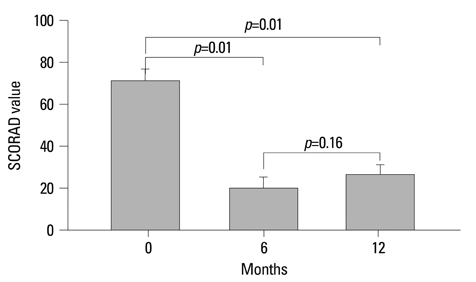
In 8 patients who completed 12 months of treatment, the SCORAD values significantly decreased from 71.5+/-15.5 (mean+/-SD) at baseline to 20.4+/-14.6 at 6 months and 26.3+/-13.6 at 12 months (Wilcoxon signed-rank test, p=0.01), and no significant systemic side effects were observed. Cyclosporin was discontinued in 4 of 8 patients within 8 months after starting the combined treatment.
CONCLUSION
In this study, combined treatment with SCIT and cyclosporin resulted in significant clinical improvements in patients with severe AD. Further studies are needed to test the clinical usefulness of this combined treatment for patients with severe AD.
Keyword
MeSH Terms
-
Adolescent
Adult
Allergens/*administration & dosage
Combined Modality Therapy
Cyclosporine/*administration & dosage
Dermatitis, Atopic/*drug therapy/immunology
Desensitization, Immunologic/*methods
Female
Humans
Immunosuppressive Agents/*administration & dosage
Injections, Subcutaneous
Male
Severity of Illness Index
Treatment Outcome
Figure
Cited by 3 articles
-
Production of Egg Yolk Antibodies Specific to House Dust Mite Proteins
Kyung Eun Lee, Beom Ku Han, Jae Yong Han, Jung Yeon Hong, Mi Na Kim, Won Il Heo, Myung Hyun Sohn, Kyung Won Kim, Kyu Earn Kim
Yonsei Med J. 2014;55(4):999-1004. doi: 10.3349/ymj.2014.55.4.999.Clinical Efficacy of Subcutaneous Allergen Immunotherapy in Patients with Atopic Dermatitis
Dong-Ho Nahm, Myoung-Eun Kim, Byul Kwon, Su-Mi Cho, Areum Ahn
Yonsei Med J. 2016;57(6):1420-1426. doi: 10.3349/ymj.2016.57.6.1420.Treatment of Patients with Refractory Atopic Dermatitis Sensitized to House Dust Mites by Using Sublingual Allergen Immunotherapy
Joon-Seok Choi, Ha-Ryeong Ryu, Cheol-Hyun Yoon, Ji-Hoon Kim, Jin-Ok Baek, Joo-Young Roh, Jong-Rok Lee
Ann Dermatol. 2015;27(1):82-86. doi: 10.5021/ad.2015.27.1.82.
Reference
-
1. Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association "Administrative Regulations for Evidence-Based Clinical Practice Guidelines". J Am Acad Dermatol. 2004. 50:391–404.2. Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003. 112:S128–S139.
Article3. Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006. 118:152–169.
Article4. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998. 102:558–562.
Article5. Pipet A, Botturi K, Pinot D, Vervloet D, Magnan A. Allergen-specific immunotherapy in allergic rhinitis and asthma. Mechanisms and proof of efficacy. Respir Med. 2009. 103:800–812.
Article6. Novak N. Allergen specific immunotherapy for atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007. 7:542–546.
Article7. Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006. 61:202–205.
Article8. Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen-specific immunotherapy and a histamine-immunoglobulin complex. Int Arch Allergy Immunol. 2008. 146:235–240.
Article9. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980. 92:Suppl. 44–47.10. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007. 157:645–648.
Article11. Kim ME, Kim JE, Sung JM, Lee JW, Choi GS, Nahm DH. Safety of accelerated schedules of subcutaneous allergen immunotherapy with house dust mite extract in patients with atopic dermatitis. J Korean Med Sci. 2011. 26:1159–1164.
Article12. BuBmann C, Bieber T, Novak N. Systemic therapeutic options for severe atopic dermatitis. J Dtsch Dermatol Ges. 2009. 7:205–219.
Article13. Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2000. CD001186.
Article14. Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007. CD001936.
Article15. Czarnecka-Operacz M, Silny W. Specific immunotherapy in atopic dermatitis--Four-year treatment in different age and airborne allergy type subgroups. Acta Dermatovenerol Croat. 2006. 14:230–240.16. Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema - a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2007. 21:606–619.
Article17. Kroot EJ, Huisman AM, Van Zeben J, Wouters JM, Van Paassen HC. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: a pilot study. Ann N Y Acad Sci. 2006. 1069:300–306.
Article18. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011. 127:18–27.
Article19. Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005. 53:338–340.
Article20. Takiguchi R, Tofte S, Simpson B, Harper E, Blauvelt A, Hanifin J, et al. Efalizumab for severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2007. 56:222–227.
Article21. Simon D, Hösli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008. 121:122–128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex
- Allergen-specific Immunotherapy in Patients with Atopic Dermatitis
- A Clinical Experience of Cyclosporin A in Severe Atopic Dermatitis of Children
- Safety of Accelerated Schedules of Subcutaneous Allergen Immunotherapy with House Dust Mite Extract in Patients with Atopic Dermatitis
- Treatment of Patients with Refractory Atopic Dermatitis Sensitized to House Dust Mites by Using Sublingual Allergen Immunotherapy