Yonsei Med J.
2012 Jan;53(1):83-90. 10.3349/ymj.2012.53.1.83.
Early Experience Using a Left Atrial Appendage Occlusion Device in Patients with Atrial Fibrillation
- Affiliations
-
- 1Department of Cardiology, Yonsei University Health System, Seoul, Korea. hnpak@yuhs.ac
- 2Department of Cardiology, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea.
- 3Department of Cardiology, Korea University Cardiovascular Center, Seoul, Korea.
- KMID: 1779690
- DOI: http://doi.org/10.3349/ymj.2012.53.1.83
Abstract
- PURPOSE
Atrial fibrillation (AF) is one of the major risk factors for ischemic stroke, and 90% of thromboembolisms in these patients arise from the left atrial appendage (LAA). Recently, it has been documented that an LAA occlusion device (OD) is not inferior to warfarin therapy, and that it reduces mortality and risk of stroke in patients with AF.
MATERIALS AND METHODS
We implanted LAA-ODs in 5 Korean patients (all male, 59.8+/-7.3 years old) with long-standing persistent AF or permanent AF via a percutaneous trans-septal approach.
RESULTS
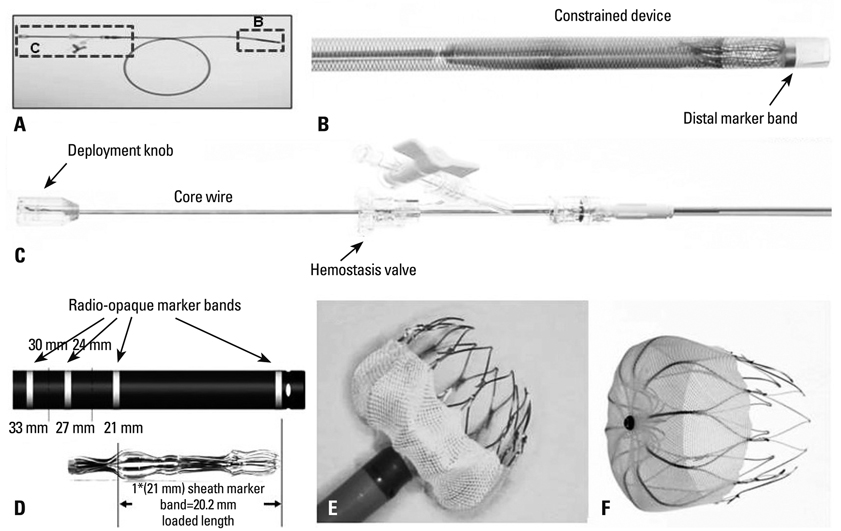
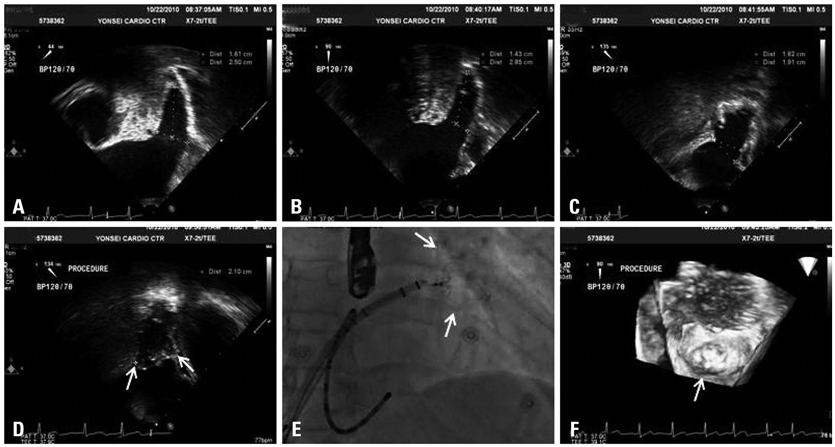
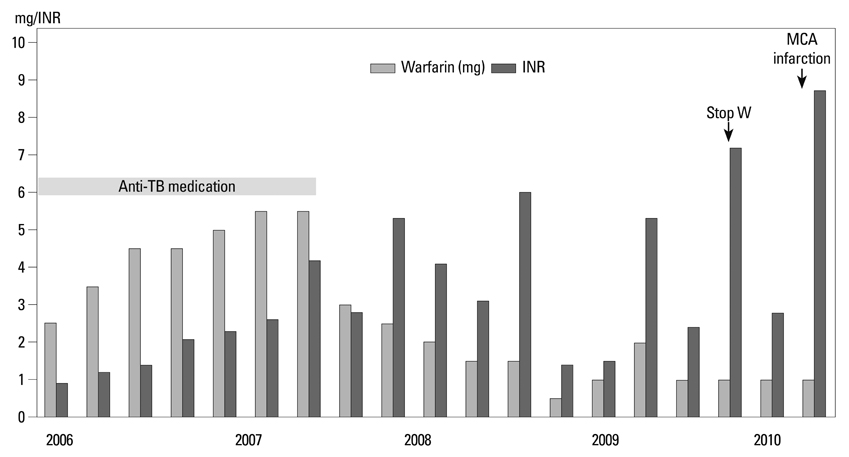
1) The major reasons for LAA-OD implantation were high risk of recurrent stroke (80%), labile international neutralizing ratio with hemorrhage (60%), and 3/5 (60%) patients had a past history of failed cardioversion for rhythm control. 2) The mean LA size was 51.3+/-5.0 mm and LAA size was 25.1x30.1 mm. We implanted the LAA-OD (28.8+/-3.4 mm device) successfully in all 5 patients with no complications. 3) After eight weeks of anticoagulation, all patients switched from warfarin to anti-platelet agent after confirmation of successful LAA occlusion by trans-esophageal echocardiography.
CONCLUSION
We report on our early experience with LAA-OD deployment in patients with 1) persistent or permanent AF who cannot tolerate anticoagulation despite significant risk of ischemic stroke, or 2) recurrent stroke in patients who are unable to maintain sinus rhythm.
MeSH Terms
Figure
Cited by 2 articles
-
Characteristics of Pulmonary Vein Enlargement in Non-Valvular Atrial Fibrillation Patients with Stroke
Jung Myung Lee, Jong-Youn Kim, Jaemin Shim, Jae-Sun Uhm, Young Jin Kim, Hye-Jeong Lee, Hui-Nam Pak, Moon-Hyoung Lee, Boyoung Joung
Yonsei Med J. 2014;55(6):1516-1525. doi: 10.3349/ymj.2014.55.6.1516.Simultaneous Closure of a Left Atrial Appendage through an Atrial Septal Defect and the Atrial Septal Defect
Shinjeong Song, Oh-Hyun Lee, Jung-Sun Kim, In-Jeong Cho, Chi-Young Shim, Geu-Ru Hong, Hui-Nam Pak, Yangsoo Jang
Yonsei Med J. 2017;58(6):1237-1240. doi: 10.3349/ymj.2017.58.6.1237.
Reference
-
1. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982. 306:1018–1022.
Article2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001. 285:2370–2375.
Article3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991. 22:983–988.
Article4. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998. 98:946–952.5. Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995. 25:452–459.
Article6. Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med. 1995. 123:817–822.
Article7. Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994. 24:755–762.
Article8. Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009. 151:297–305.
Article9. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004. 109:1509–1513.
Article10. Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989. 1:175–179.11. Hylek EM, D'Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006. 37:1075–1080.
Article12. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009. 361:1139–1151.
Article13. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006. 367:1903–1912.
Article14. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999. 131:927–934.
Article15. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010. 12:1360–1420.16. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008. 118:e523–e661.17. Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005. 150:288–293.
Article18. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009. 374:534–542.
Article19. Hwang JJ, Ko FN, Li YH, Ma HM, Wu GJ, Chang H, et al. Clinical implications and factors related to left atrial spontaneous echo contrast in chronic nonvalvular atrial fibrillation. Cardiology. 1994. 85:69–75.
Article20. Blackshear JL, Johnson WD, Odell JA, Baker VS, Howard M, Pearce L, et al. Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J Am Coll Cardiol. 2003. 42:1249–1252.
Article21. Block PC, Burstein S, Casale PN, Kramer PH, Teirstein P, Williams DO, et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009. 2:594–600.
Article22. Singh SM, Dukkipati SR, d'Avila A, Doshi SK, Reddy VY. Percutaneous left atrial appendage closure with an epicardial suture ligation approach: a prospective randomized pre-clinical feasibility study. Heart Rhythm. 2010. 7:370–376.
Article23. Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011. 123:417–424.
Article24. Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007. 6:981–993.
Article25. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009. 360:668–678.
Article26. Joung B, Chen PS, Lin SF. The role of the calcium and the voltage clocks in sinoatrial node dysfunction. Yonsei Med J. 2011. 52:211–219.
Article27. Kim WH, Joung B, Shim J, Park JS, Hwang ES, Pak HN, et al. Long-term outcome of single-chamber atrial pacing compared with dual-chamber pacing in patients with sinus-node dysfunction and intact atrioventricular node conduction. Yonsei Med J. 2010. 51:832–837.
Article28. ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010. 159:340–347.e1.29. Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011. 77:700–706.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of Electrical Cardioversion for Atrial Fibrillation on Left Atrial Appendage Function: A Transesophageal Echocardiography Study
- Simultaneous Closure of a Left Atrial Appendage through an Atrial Septal Defect and the Atrial Septal Defect
- Delayed Sealing of WATCHMAN Device Shunt
- Left Atrial Appendages Occlusion: Current Status and Prospective
- Persistent Atrial Fibrillation Related to a Congenital Pericardial Defect and Left Atrial Appendage Herniation