Korean Circ J.
2018 Aug;48(8):692-704. 10.4070/kcj.2018.0231.
Left Atrial Appendages Occlusion: Current Status and Prospective
- Affiliations
-
- 1Kansas City Heart Rhythm Institute, Overland Park, KS, USA. dlakkireddy@kchrf.org
- KMID: 2417692
- DOI: http://doi.org/10.4070/kcj.2018.0231
Abstract
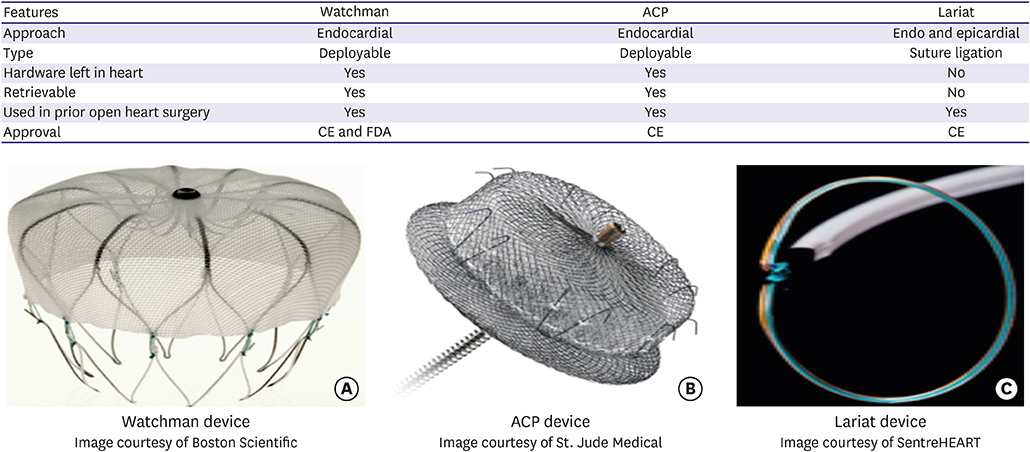
- Stroke continues to be a major cause of morbidity and mortality in atrial fibrillation (AF) patients. Oral anticoagulation (OAC) provides protection against stroke and peripheral embolization in AF but significant proportion of patients could not be started on anticoagulation because of bleeding complications. Left atrial appendage harbors clot in about 90% of nonvalvular AF. The advent of left atrial appendage occlusion (LAAO) techniques has provided these patients with alternative to OAC for stroke prophylaxis. Multiple LAAO devices are currently available with Watchman and Amulet being the most commonly used in clinical practice. Randomized studies are available for Watchman device only. Data on Amplatzer Cardiac Plug, Amulet and Lariat devices are limited by the paucity of randomized data. Long-term data on different LAAO techniques are showing promising results. Device related thrombosis continues to be a serious complication associated with LAAO. Future studies should look into comparative effectiveness between different LAAO techniques, optimal patient selection, risk of complications, and anticoagulant treatment after LAAO. This article aims to provide current available evidence on efficacy and safety of different LAAO devices and future prospective.
MeSH Terms
Figure
Reference
-
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375.2. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119–125.
Article3. Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008; 31:55–62.
Article4. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867.
Article5. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007; 370:493–503.
Article6. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996; 61:755–759.
Article7. Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001; 344:1411–1420.
Article8. Onalan O, Crystal E. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke. 2007; 38:624–630.
Article9. U.S. Food and Drug Administration. Watchman LAA closure technology -P130013 [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2015. cited 2018 May 17. Available form: https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130013a.pdf.10. Holmes DR, Reddy VY, Turi ZG, et al. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009; 374:534–542.
Article11. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014; 64:1–12.12. Reddy VY, Gibson DN, Kar S, et al. Post-approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017; 69:253–261.
Article13. Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011; 123:417–424.14. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015; 65:2614–2623.15. Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016; 37:2465–2474.
Article16. Betts TR, Leo M, Panikker S, et al. Percutaneous left atrial appendage occlusion using different technologies in the United Kingdom: a multicenter registry. Catheter Cardiovasc Interv. 2017; 89:484–492.
Article17. Alli O, Doshi S, Kar S, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013; 61:1790–1798.
Article18. Reddy VY, Akehurst RL, Armstrong SO, et al. Cost effectiveness of left atrial appendage closure with the Watchman device for atrial fibrillation patients with absolute contraindications to warfarin. Europace. 2016; 18:979–986.
Article19. Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR Jr. Time to cost-effectiveness following stroke reduction strategies in AF: warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015; 66:2728–2739.20. Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016; 11:1170–1179.21. Santoro G, Meucci F, Stolcova M, et al. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention. 2016; 11:1188–1194.
Article22. Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017; 13:867–876.
Article23. Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013; 62:96–102.
Article24. Berti S, Pastormerlo LE, Rezzaghi M, et al. Left atrial appendage occlusion in high-risk patients with non-valvular atrial fibrillation. Heart. 2016; 102:1969–1973.
Article25. Koskinas KC, Shakir S, Fankhauser M, et al. Predictors of early (1-week) outcomes following left atrial appendage closure with Amplatzer devices. JACC Cardiovasc Interv. 2016; 9:1374–1383.
Article26. Kleinecke C, Park JW, Gödde M, Zintl K, Schnupp S, Brachmann J. Twelve-month follow-up of left atrial appendage occlusion with Amplatzer Amulet. Cardiol J. 2017; 24:131–138.
Article27. López Mínguez JR, Asensio JM, Gragera JE, et al. Two-year clinical outcome from the Iberian registry patients after left atrial appendage closure. Heart. 2015; 101:877–883.
Article28. Gloekler S, Shakir S, Doblies J, et al. Early results of first versus second generation Amplatzer occluders for left atrial appendage closure in patients with atrial fibrillation. Clin Res Cardiol. 2015; 104:656–665.
Article29. Abualsaud A, Freixa X, Tzikas A, et al. Side-by-side comparison of LAA occlusion performance with the Amplatzer cardiac plug and Amplatzer Amulet. J Invasive Cardiol. 2016; 28:34–38.30. Genovesi S, Slaviero G, Porcu L, et al. Implant success and safety of left atrial appendage occlusion in end stage renal disease patients: peri-procedural outcomes from an Italian dialysis population. Int J Cardiol. 2018; 262:38–42.
Article31. Lee OH, Kim JS, Pak HN, et al. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol. 2018; 121:1534–1539.
Article32. Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016; 13:1030–1036.
Article33. Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013; 62:108–118.34. Price MJ, Gibson DN, Yakubov SJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014; 64:565–572.35. Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg . 2011; 142:1002–1009. 1009.e1
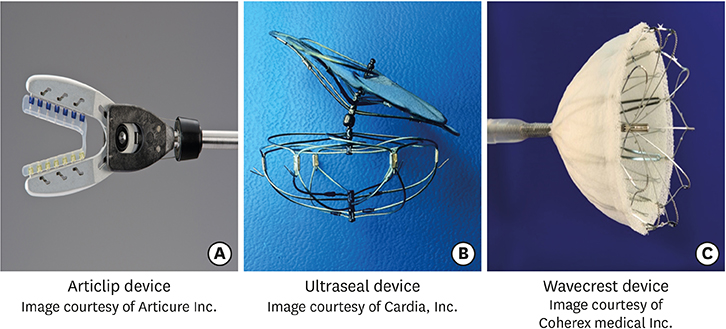
Article36. Regueiro A, Bernier M, O'Hara G, et al. Left atrial appendage closure: initial experience with the ultraseal device. Catheter Cardiovasc Interv. 2017; 90:817–823.
Article37. Coherex WAVECREST I left atrial appendage occlusion study [Internet]. Bethesda (MD): U.S. National Library of Medicine;2015. cited 2018 May 15. Available form: https://clinicaltrials.gov/ct2/show/NCT02239887?cond=WAVECREST&rank=1.38. Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015; 47:847–854.
Article39. Fauchier L, Cinaud A, Brigadeau F, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018; 71:1528–1536.40. Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007; 49:1490–1495.
Article41. Main ML, Fan D, Reddy VY, et al. Assessment of device-related thrombus and associated clinical outcomes with the Watchman left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the PROTECT-AF trial). Am J Cardiol. 2016; 117:1127–1134.
Article42. Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol. 2013; 61:2551–2556.43. Meincke F, Schmidt-Salzmann M, Kreidel F, Kuck KH, Bergmann MW. New technical and anticoagulation aspects for left atrial appendage closure using the WATCHMAN® device in patients not taking warfarin. EuroIntervention. 2013; 9:463–468.
Article44. Bösche LI, Afshari F, Schöne D, Ewers A, Mügge A, Gotzmann M. Initial experience with novel oral anticoagulants during the first 45 days after left atrial appendage closure with the Watchman device. Clin Cardiol. 2015; 38:720–724.
Article45. Enomoto Y, Gadiyaram VK, Gianni C, et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017; 14:19–24.
Article46. Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017; 70:2964–2975.47. López-Mínguez JR, Nogales-Asensio JM, Infante De Oliveira E, et al. Long-term event reduction after left atrial appendage closure. Results of the Iberian Registry II. Rev Esp Cardiol (Engl Ed). 2018; S1885-5857(18)30112-9.
Article48. Nietlispach F, Gloekler S, Krause R, et al. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013; 82:283–289.
Article49. Regueiro A, Cruz-Gonzalez I, Bethencourt A, et al. Long-term outcomes following percutaneous left atrial appendage closure in patients with atrial fibrillation and contraindications to anticoagulation. J Interv Card Electrophysiol. 2018; 52:53–59.
Article50. Pillarisetti J, Reddy YM, Gunda S, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm. 2015; 12:1501–1507.
Article51. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014; 130:2071–2104.52. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016; 18:1609–1678.
Article53. Cruz-Gonzalez I, Palazuelos Molinero J, Valenzuela M, et al. Brain natriuretic peptide levels variation after left atrial appendage occlusion. Catheter Cardiovasc Interv. 2016; 87:E39–43.
Article54. Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the Watchman device. J Invasive Cardiol. 2015; 27:448–452.55. Lakkireddy D, Turagam M, Afzal MR, et al. Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol. 2018; 71:135–144.56. Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm. 2015; 12:52–59.
Article57. Badhwar N, Lakkireddy D, Kawamura M, et al. Sequential percutaneous LAA ligation and pulmonary vein isolation in patients with persistent AF: initial results of a feasibility study. J Cardiovasc Electrophysiol. 2015; 26:608–614.
Article58. Starck CT, Steffel J, Emmert MY, et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012; 15:416–418.
Article59. aMAZE Study: LAA ligation adjunctive to PVI for persistent or longstanding persistent atrial fibrillation (aMAZE) [Internet]. Bethesda (MD): U.S. National Library of Medicine;2018. cited 2018 May 19. Available from: https://clinicaltrials.gov/ct2/show/NCT02513797.60. AMPLATZERTM AmuletTM LAA Occluder trial (Amulet IDE) [Internet]. Bethesda (MD): U.S. National Library of Medicine;2018. cited 2018 May 17. Available form: https://clinicaltrials.gov/ct2/show/NCT02879448?cond=Amplatzer&draw=2&rank=1.61. Left atrial appendage closure vs. novel anticoagulation agents in atrial fibrillation (PRAGUE-17) [Internet]. Bethesda (MD): U.S. National Library of Medicine;2016. cited 2018 May 17. Available form: https://clinicaltrials.gov/ct2/show/NCT02426944?cond=PRAGUE+17&rank=1.62. Assessment of the WATCHMANTM Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) [Internet]. Bethesda (MD): U.S. National Library of Medicine;2018. cited 2018 May 17. Available form: https://clinicaltrials.gov/ct2/show/NCT02928497?cond=ASAP+TOO&rank=1.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Developmental Morphology of the Atrial Venous Component in the iv/iv Mouse
- Percutaneous Left Atrial Appendage Occlusion for the Prevention of Stroke in Patients with Atrial Fibrillation: Review and Critical Appraisal
- Simultaneous Closure of a Left Atrial Appendage through an Atrial Septal Defect and the Atrial Septal Defect
- Heterotaxy Syndrome
- Cardioembolic Stroke in Atrial Fibrillation-Rationale for Preventive Closure of the Left Atrial Appendage