Yonsei Med J.
2011 Mar;52(2):326-332. 10.3349/ymj.2011.52.2.326.
Comparison of the Effects of Propofol and Midazolam on Inflammation and Oxidase Stress in Children with Congenital Heart Disease Undergoing Cardiac Surgery
- Affiliations
-
- 1Department of Critic Care Medicine, Renmin Hospital of Wuhan University, Wuhan, P.R. China. zhouqingshan2010@yahoo.com.cn
- 2Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, P.R. China.
- 3Cardiovascular Research Institute, Wuhan University, Wuhan, P.R. China.
- KMID: 1779671
- DOI: http://doi.org/10.3349/ymj.2011.52.2.326
Abstract
- PURPOSE
To investigate and compare the effects of propofol and midazolam on inflammation and oxidase stress in children with congenital heart disease undergoing cardiac surgery.
MATERIALS AND METHODS
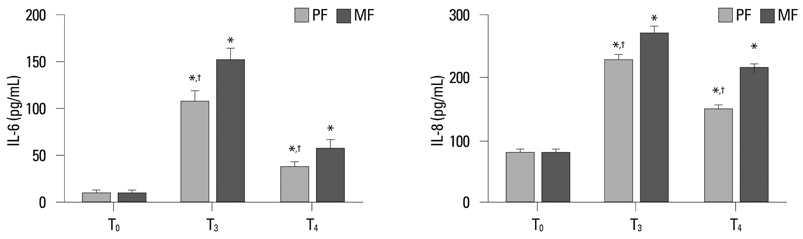
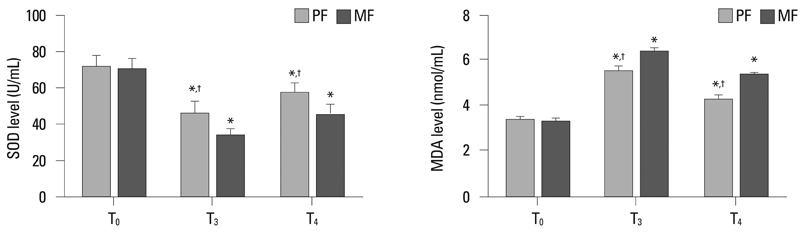
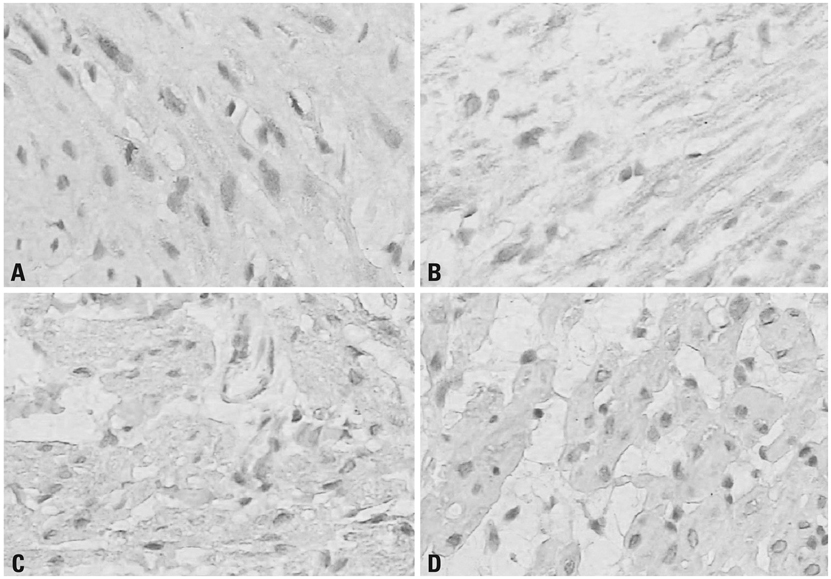
Thirty-two ASA class I-II children with congenital heart disease undergoing cardiac surgery were randomly divided into two groups: propofol combined with low dose fentanyl (PF group, n = 16) and midazolam combined with low dose fentanyl (MF group, n = 16). Tracheal extubation time and length of Intensive Care Unit (ICU) stay were recorded. Blood samples were taken before operation (T0), at 2 h after release of the aorta cross-clamp (T3) and at 24 h after operation (T4) to measure interleukin 6 (IL-6), IL-8, superoxide dismutase (SOD) and malondialdehyde (MDA) levels. Myocardium samples were collected at 10-20 min after aorta cross-clamp (T1) and at 10-20 min after the release of the aorta cross-clamp (T2) to detect heme oxygenase-1 (HO-1) expression.
RESULTS
Tracheal extubation time and length of ICU stay in PF group were significantly shorter than those of the MF group (p < 0.05, respectively). After cardiopulmonary bypass, IL-6, IL-8 and MDA levels were significantly increased, and the SOD level was significantly reduced in both two groups, but PF group exhibited lower IL-6, IL-8 and MDA levels and higher SOD levels than the MF group (p < 0.05, respectively). The HO-1 expression in the PF group was significantly higher than that in MF group at the corresponding time points (p < 0.05, respectively).
CONCLUSION
Propofol is superior to midazolam in reducing inflammation and oxidase stress and in improving post-operation recovery in children with congenital heart disease undergoing cardiac surgery.
Keyword
MeSH Terms
-
Anesthesia, Intravenous/*adverse effects
Anesthetics, Intravenous/*adverse effects
Cardiac Surgical Procedures/*adverse effects
Child
Female
Heart Defects, Congenital/*surgery
Heme Oxygenase-1/blood
Humans
Inflammation/*chemically induced
Interleukin-6/blood
Interleukin-8/blood
Male
Malondialdehyde/blood
Midazolam/*adverse effects
Oxidative Stress/*drug effects
Propofol/*adverse effects
Superoxide Dismutase/blood
Figure
Reference
-
1. Sadowski SL. Congenital cardiac disease in the newborn infant: past, present, and future. Crit Care Nurs Clin North Am. 2009. 21:37–48.
Article2. Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res. 2005. 57:22–27.
Article3. Stark J, Gallivan S, Lovegrove J, Hamilton JR, Monro JL, Pollock JC, et al. Mortality rates after surgery for congenital heart defects in children and surgeons' performance. Lancet. 2000. 355:1004–1007.
Article4. Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009. 102:240–247.
Article5. Xia Z, Godin DV, Chang TK, Ansley DM. Dose-dependent protection of cardiac function by propofol during ischemia and early reperfusion in rats: effects on 15-F2t-isoprostane formation. Can J Physiol Pharmacol. 2003. 81:14–21.
Article6. Corcoran TB, Engel A, Sakamoto H, O'Shea A, O'Callaghan-Enright S, Shorten GD. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. Br J Anaesth. 2006. 97:825–831.
Article7. Bartosikova L, Necas J, Bartosik T, Frana P, Pavlik M. Changes in biomechanical parameters during heart perfusion and after midazolam pre-medication--experimental pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008. 152:79–82.
Article8. Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, et al. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann N Y Acad Sci. 2005. 1042:262–271.
Article9. Kim SN, Son SC, Lee SM, Kim CS, Yoo DG, Lee SK, et al. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006. 105:105–110.
Article10. Kang MY, Tsuchiya M, Packer L, Manabe M. In vitro study on antioxidant potential of various drugs used in the perioperative period. Acta Anaesthesiol Scand. 1998. 42:4–12.
Article11. Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009. 32:4–16.
Article12. Blancke F, Claeys MJ, Jorens P, Vermeiren G, Bosmans J, Wuyts FL, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005. 2005:385–389.
Article13. Ji L, Fu F, Zhang L, Liu W, Cai X, Zhang L, et al. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/ nitrative stress. Am J Physiol Endocrinol Metab. 2010. 298:E871–E880.14. Prabhu A, Sujatha DI, Kanagarajan N, Vijayalakshmi MA, Ninan B. Effect of N-acetylcysteine in attenuating ischemic reperfusion injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg. 2009. 23:645–651.
Article15. Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998. 86:252–258.
Article16. De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg. 1999. 89:1050–1055.
Article17. Tsuchiya M, Asada A, Maeda K, Ueda Y, Sato EF, Shindo M, et al. Propofol versus midazolam regarding their antioxidant activities. Am J Respir Crit Care Med. 2001. 163:26–31.
Article18. An K, Shu H, Huang W, Huang X, Xu M, Yang L, et al. Effects of propofol on pulmonary inflammatory response and dysfunction induced by cardiopulmonary bypass. Anaesthesia. 2008. 63:1187–1192.19. Zavala F, Taupin V, Descamps-Latscha B. In vivo treatment with benzodiazepines inhibits murine phagocyte oxidative metabolism and production of interleukin 1, tumor necrosis factor and interleukin-6. J Pharmacol Exp Ther. 1990. 255:442–450.20. Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998. 86:159–165.21. Joo HK, Oh SC, Cho EJ, Park KS, Lee JY, Lee EJ, et al. Midazolam inhibits tumor necrosis factor-alpha-induced endothelial activation: involvement of the peripheral benzodiazepine receptor. Anesthesiology. 2009. 110:106–112.22. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006. 86:583–650.
Article23. Tsuchihashi S, Fondevila C, Kupiec-Weglinski JW. Heme oxygenase system in ischemia and reperfusion injury. Ann Transplant. 2004. 9:84–87.24. Burger D, Xiang F, Hammoud L, Lu X, Feng Q. Role of heme oxygenase-1 in the cardioprotective effects of erythropoietin during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2009. 296:H84–H93.
Article25. Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001. 89:168–173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propofol versus Midazolam for Sedation during Esophagogastroduodenoscopy in Children
- Comparison of Midazolam Alone versus Midazolam Plus Propofol during Endoscopic Submucosal Dissection
- The Effects of Midazolam and Propofol by Continuous Intravenous Infusion to provide Sedation in Patients who receive Spinal Anesthesia
- Effect of Midazolam, Fentanyl and Propofol for Intravenous Anesthesia in Patients Undergoing the Cardioversion
- Effects of Midazolam and Propofol as Sedative Agents on Cardiopulmonary Function under Spinal Anesthesia