Yonsei Med J.
2011 Mar;52(2):301-306. 10.3349/ymj.2011.52.2.301.
Comparison of Diagnostic Performance of Three Real-Time PCR Kits for Detecting Mycobacterium Species
- Affiliations
-
- 1Department of Laboratory Medicine, Kyung Hee University School of Medicine, Seoul, Korea. leehejo@khmc.or.kr
- KMID: 1779667
- DOI: http://doi.org/10.3349/ymj.2011.52.2.301
Abstract
- PURPOSE
PCR is widely used for rapidly and accurately detecting Mycobacterium Species. The purpose of this study was to assess the diagnostic performance of three real-time PCR kits and evaluate the concordance with two older PCR methods.
MATERIALS AND METHODS
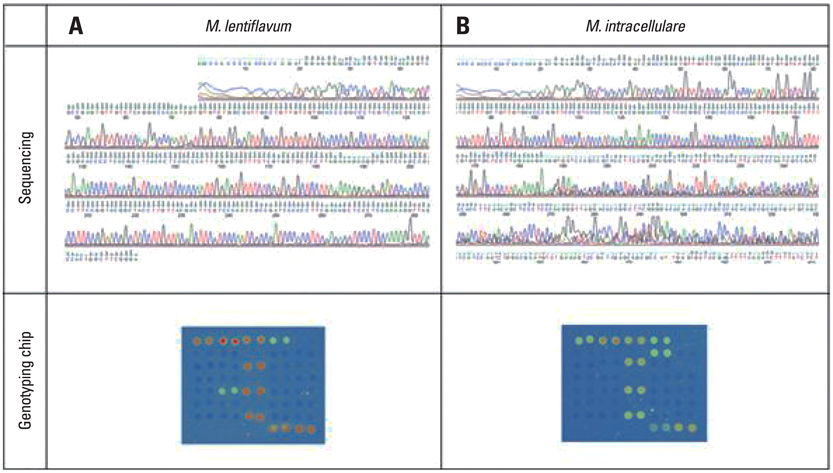
Using 128 samples, the five PCR methods were assessed, including an in-house PCR protocol, the COBAS Amplicor MTB, the COBAS TaqMan MTB, the AdvanSure TB/NTM real-time PCR, and the Real-Q M. tuberculosis kit. The discrepant results were further examined by DNA sequencing and using the AdvanSure Mycobacteria Genotyping Chip for complete analysis.
RESULTS
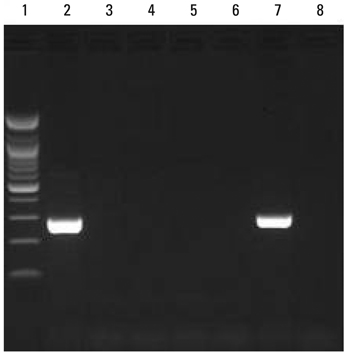
For Mycobacterium tuberculosis (MTB) detection, all five kits showed 100% matching results (positive; N = 11 and negative; N = 80). In non-tuberculous mycobacterium (NTM) discrimination, the AdvanSure yielded two true-positive outcomes from M. intracellulare and one false positive outcome, while the Real-Q resulted in one true-positive outcome and one false negative outcome for each case and another false negative result using the provided DNA samples.
CONCLUSION
Real-time PCR, yielded results that were comparable to those of the older PCR methods for detecting MTB. However, there were disagreements among the applied kits in regard to the sample test results for detecting NTM. Therefore, we recommend that additional confirmatory measures such as DNA sequencing should be implemented in such cases, and further research with using a larger numbers of samples is warranted to improve the detection of NTM.
MeSH Terms
-
DNA, Bacterial/genetics
Humans
Mycobacterium/*genetics
Mycobacterium Infections/*diagnosis/microbiology
Mycobacterium avium Complex/genetics
Mycobacterium avium-intracellulare Infection/diagnosis
Mycobacterium tuberculosis/genetics
Polymerase Chain Reaction/*standards
Reagent Kits, Diagnostic/*standards
Tuberculosis/diagnosis
Figure
Reference
-
1. World Health Organization. New technologies for tuberculosis control: a framework for their adoption, introduction and implementation. World Health Organization, 2007. Appia, Geneva, Switzerland: WHO Press.2. Chang HE, Heo SR, Yoo KC, Song SH, Kim SH, Kim HB, et al. [Detection of Mycobacterium tuberculosis complex using real-time polymerase chain reaction]. Korean J Lab Med. 2008. 28:103–108.
Article3. Kang SH, Yoo KC, Park KU, Song J, Kim EC. [Usefulness of multiplex real-Time PCR and melting curve analysis in identification of nontuberculous mycobacteria]. Korean J Lab Med. 2007. 27:40–45.
Article4. Baba K, Pathak S, Sviland L, Langeland N, Hoosen AA, Asjo B, et al. Real-time quantitative PCR in the diagnosis of tuberculosis in formalin-fixed paraffin-embedded pleural tissue in patients from a high HIV endemic area. Diagn Mol Pathol. 2008. 17:112–117.
Article5. Lee MF, Chen YH, Peng CF. Evaluation of reverse transcription loop-mediated isothermal amplification in conjunction with ELISA-hybridization assay for molecular detection of Mycobacterium tuberculosis. J Microbiol Methods. 2009. 76:174–180.
Article6. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000. 38:2966–2971.
Article7. Flores E, Rodríguez JC, Garcia-Pachón E, Soto JL, Ruiz M, Escribano I, et al. Real-time PCR with internal amplification control for detecting tuberculosis: method design and validation. APMIS. 2009. 117:592–597.
Article8. Richardson ET, Samson D, Banaei N. Rapid Identification of Mycobacterium tuberculosis and nontuberculous mycobacteria by multiplex, real-time PCR. J Clin Microbiol. 2009. 47:1497–1502.
Article9. Mackay IM. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004. 10:190–212.
Article10. Tortoli E, Tronci M, Tosi CP, Galli C, Lavinia F, Natili S, et al. Multicenter evaluation of two commercial amplification kits (Amplicor, Roche and LCx, Abbott) for direct detection of Mycobacterium tuberculosis in pulmonary and extrapulmonary specimens. Diagn Microbiol Infect Dis. 1999. 33:173–179.
Article11. Yang HY, Lee HJ, Park SY, Lee KK, Suh JT. [Comparison of In-house Polymerase Chain Reaction Assay with Conventional Techniques for the Detection of Mycobacterium tuberculosis]. Korean J Lab Med. 2006. 26:174–178.
Article12. Choi YM, Lee MH. Detection of Mycobacterium tuberculosis in sputum by using polymerase chain reaction. Korean J Clin Microbiol. 1999. 2:144–151.13. Kim YJ, Park MY, Kim SY, Cho SA, Hwang SH, Kim HH, et al. [Evaluation of the performances of advanSure TB/NTM real time PCR Kit for detection of mycobacteria in respiratory specimens]. Korean J Lab Med. 2008. 28:34–38.
Article14. Jung CL, Kim MK, Seo DC, Lee MA. Clinical usefulness of real-time PCR and amplicor MTB PCR assays for diagnosis of tuberculosis. Korean J Clin Microbiol. 2008. 11:29–33.
Article15. Scarparo C, Piccoli P, Rigon A, Ruggiero G, Scagnelli M, Piersimoni C. Comparison of enhanced Mycobacterium tuberculosis amplified direct test with COBAS AMPLICOR Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J Clin Microbiol. 2000. 38:1559–1562.
Article16. Goessens WH, de Man P, Koeleman JG, Luijendijk A, te Witt R, Endtz HP, et al. Comparison of the COBAS AMPLICOR MTB and BDProbeTec ET assays for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2005. 43:2563–2566.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Anyplex plus MTB/NTM and AdvanSure TB/NTM for the Detection of Mycobacterium tuberculosis and Nontuberculous Mycobacteria
- Evaluation of Two Commercial HLA-B27 Real-Time PCR Kits
- Performance Evaluation of Anyplex Plus MTB/NTM and MDR-TB Detection Kit for Detection of Mycobacteria and for Anti-Tuberculosis Drug Susceptibility Test
- Comparison of Ogawa Media, BACTEC MGIT 960 System and TB/NTM Real-Time PCR for Detecting Mycobacterium Species
- Evaluation of the Efficacies of Rapid Antigen Test, Multiplex PCR, and Real-time PCR for the Detection of a Novel Influenza A (H1N1) Virus