Yonsei Med J.
2011 Mar;52(2):268-275. 10.3349/ymj.2011.52.2.268.
Addition of Theophylline or Increasing the Dose of Inhaled Corticosteroid in Symptomatic Asthma: A Meta-Analysis of Randomized Controlled Trials
- Affiliations
-
- 1Institute of Respiratory Diseases, Xinqiao Hospital, Third Military Medical University, Chongqing, China. wangyanflower@163.com
- 2Department of Respiratory Diseases, Fuling Central Hospital, Fuling District, Chongqing, China.
- KMID: 1779662
- DOI: http://doi.org/10.3349/ymj.2011.52.2.268
Abstract
- PURPOSE
Low-dose theophylline has anti-inflammatory effects. The aim of this study was to evaluate the effects of adding theophylline compared with increasing the dose of inhaled corticosteroid (ICS) on symptomatic asthma.
MATERIALS AND METHODS
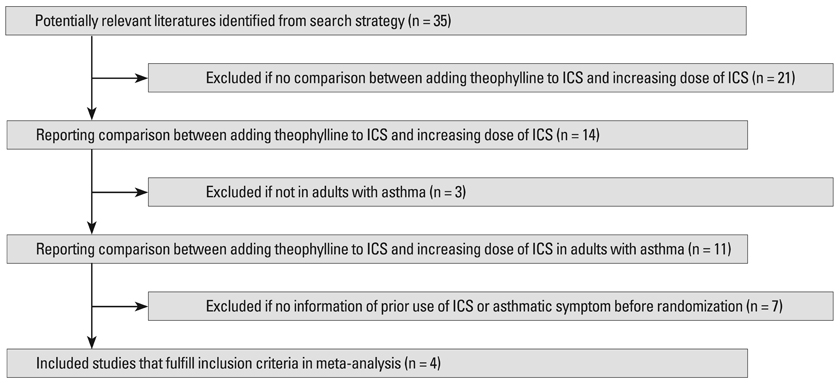
The associated literature was acquired through deliberate searching and selected based on the established inclusion criteria for publications. The extracted data were further analyzed by a meta-analysis
RESULTS
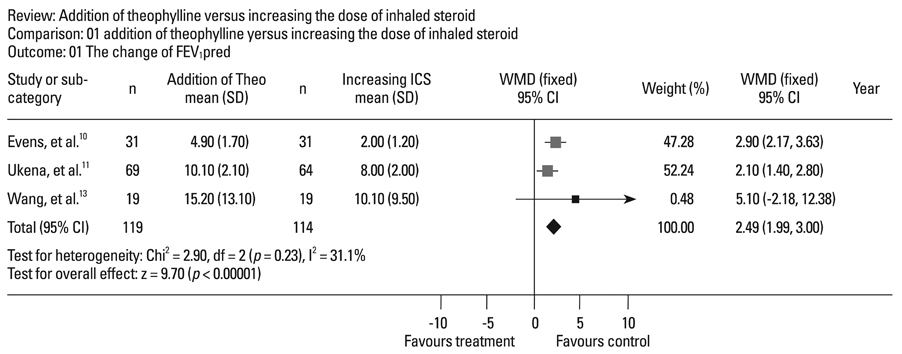
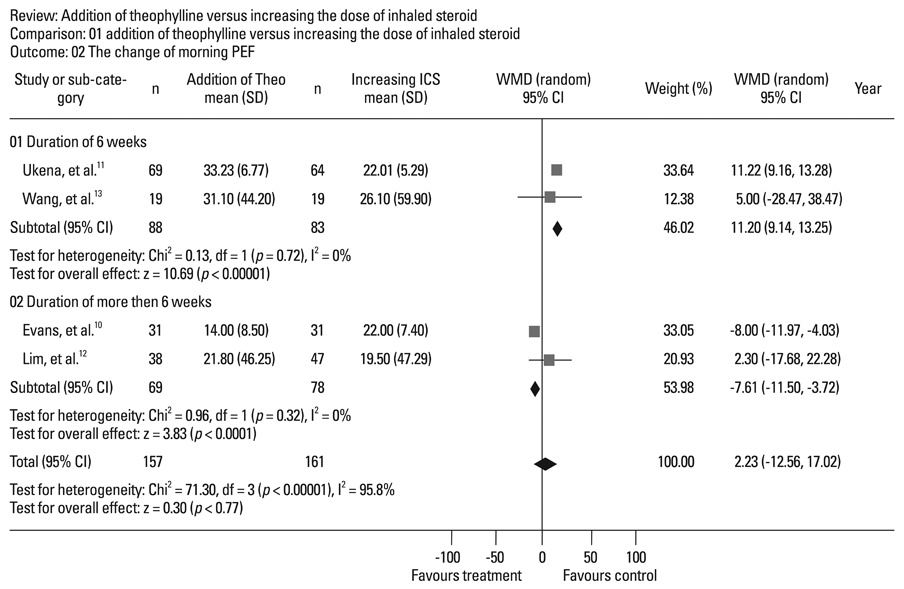
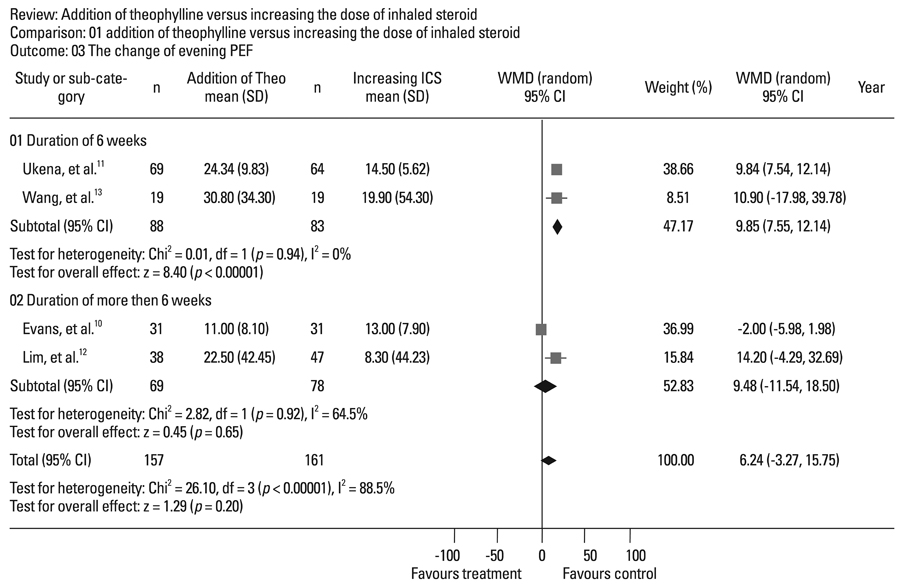
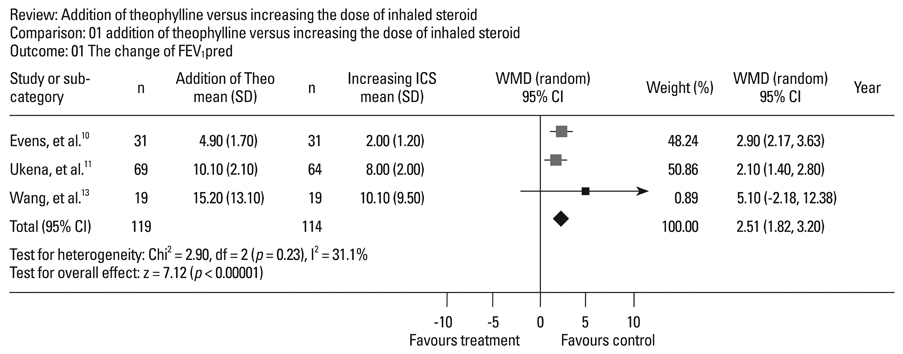
Four randomized, controlled, parallel studies were selected. Addition of theophylline produced a greater increase of forced expiratory volume in one second as %predicted (FEV1pred) by 2.49% [95% confidence interval (CI) 1.99-3.00; z = 9.70; p < 0.001], compared with increasing the dose of ICS. There was no difference between the two treatments in terms of peak expiratory flow (PEF).
CONCLUSION
Addition of theophylline to ICS has similar therapeutic effects on improving lung function as increasing the dose of ICS in the treatment of symptomatic asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2009.2. Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ. 2000. 320:1368–1373.
Article3. Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006. 129:15–26.4. Weatherall M, Wijesinghe M, Perrin K, Harwood M, Beasley R. Meta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapy. Thorax. 2010. 65:39–43.
Article5. Nelson H, Bonuccelli C, Radner F, Ottosson A, Carroll KJ, Andersson TL, et al. Safety of formoterol in patients with asthma: combined analysis of data from double-blind, randomized controlled trials. J Allergy Clin Immunol. 2010. 125:390–396.
Article6. Jaeschke R, O'Byrne PM, Mejza F, Nair P, Lesniak W, Brozek J, et al. The safety of long-acting beta-agonists among patients with asthma using inhaled corticosteroids: systematic review and meta-analysis. Am J Respir Crit Care Med. 2008. 178:1009–1016.
Article7. de Vries F, Setakis E, Zhang B, van Staa TP. Long-acting {beta}2-agonists in adult asthma and the pattern of risk of death and severe asthma outcomes: a study with the GPRD. Eur Respir J. 2010. 36:494–502.
Article8. Minoguchi K, Kohno Y, Oda N, Wada K, Miyamoto M, Yokoe T, et al. Effect of theophylline withdrawal on airway inflammation in asthma. Clin Exp Allergy. 1998. 28:Suppl 3. 57–63.9. Kidney J, Dominguez M, Taylor PM, Rose M, Chung KF, Barnes PJ. Immunomodulation by theophylline in asthma. Demonstration by withdrawal therapy. Am J Respir Crit Care Med. 1995. 151:1907–1914.
Article10. Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O'Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med. 1997. 337:1412–1418.
Article11. Ukena D, Harnest U, Sakalauskas R, Magyar P, Vetter N, Steffen H, et al. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J. 1997. 10:2754–2760.
Article12. Lim S, Jatakanon A, Gordon D, Macdonald C, Chung KF, Barnes PJ. Comparison of high dose inhaled steroids, low dose inhaled steroids plus low dose theophylline, and low dose inhaled steroids alone in chronic asthma in general practice. Thorax. 2000. 55:837–841.
Article13. Wang Y, Wang CZ, Lin KX, Qian GS, Zhuo WL, Li SP, et al. Comparison of inhaled corticosteroid combined with theophylline and double-dose inhaled corticosteroid in moderate to severe asthma. Respirology. 2005. 10:189–195.
Article14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996. 17:1–12.
Article15. Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994. 343:1006–1008.
Article16. Tohda Y, Kubo H, Iwanaga T, Fukuoka M, Nakajima S. Influence of theophylline on activated lymphocytes and eosinophils in peripheral blood and sputum. J Int Med Res. 2001. 29:528–536.
Article17. Aizawa H, Iwanaga T, Inoue H, Takata S, Matsumoto K, Takahashi N, et al. Once-daily theophylline reduces serum eosinophil cationic protein and eosinophil levels in induced sputum of asthmatics. Int Arch Allergy Immunol. 2000. 121:123–128.
Article18. Jarjour NN, Lacouture PG, Busse WW. Theophylline inhibits the late asthmatic response to nighttime antigen challenge in patients with mild atopic asthma. Ann Allergy Asthma Immunol. 1998. 81:231–236.
Article19. Yao PL, Tsai MF, Lin YC, Wang CH, Liao WY, Chen JJ, et al. Global expression profiling of theophylline response genes in macrophages: evidence of airway anti-inflammatory regulation. Respir Res. 2005. 6:89.
Article20. Shute JK, Tenor H, Church MK, Holgate ST. Theophylline inhibits the release of eosinophil survival cytokines--is Raf-1 the protein kinase A target? Clin Exp Allergy. 1998. 28:Suppl 3. 47–52.21. Lim S, Tomita K, Caramori G, Jatakanon A, Oliver B, Keller A, et al. Low-dose theophylline reduces eosinophilic inflammation but not exhaled nitric oxide in mild asthma. Am J Respir Crit Care Med. 2001. 164:273–276.
Article22. Horiguchi T, Tachikawa S, Kasahara J, Doi M, Shiga M, Miyazaki J, et al. Suppression of airway inflammation by theophylline in adult bronchial asthma. Respiration. 1999. 66:124–127.
Article23. Kraft M, Torvik JA, Trudeau JB, Wenzel SE, Martin RJ. Theophylline: potential antiinflammatory effects in nocturnal asthma. J Allergy Clin Immunol. 1996. 97:1242–1246.
Article24. Cosio BG, Iglesias A, Rios A, Noguera A, Sala E, Ito K, et al. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax. 2009. 64:424–429.
Article25. Iiboshi H, Ashitani J, Katoh S, Sano A, Matsumoto N, Mukae H, et al. Long-term treatment with theophylline reduces neutrophils, interleukin-8 and tumor necrosis factor-alpha in the sputum of patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2007. 20:46–51.
Article26. Masoli M, Weatherall M, Holt S, Shirtcliffe P, Beasley R. Inhaled fluticasone propionate and adrenal effects in adult asthma: systematic review and meta-analysis. Eur Respir J. 2006. 28:960–967.
Article27. Kellerman D, Stricker W, Howland W. Effects of inhaled fluticasone propionate (FP) on the HPA axis of patients with asthma. Eur Respir J. 1996. 9:Suppl 23. 162s.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update on the Safety of Long-acting Beta2-agonists
- A Comparison of International Guidelines for Pediatric Asthma Pharmacotherapy
- Addition of Montelukast to Low-Dose Inhaled Corticosteroid Leads to Fewer Exacerbations in Older Patients Than Medium-Dose Inhaled Corticosteroid Monotherapy
- Progress in the management of childhood asthma
- Anti-Inflammatory Effect of Theophylline in Asthma Management