Korean J Radiol.
2009 Jun;10(3):277-284. 10.3348/kjr.2009.10.3.277.
In Vivo MR Imaging of Magnetically Labeled Mesenchymal Stem Cells in a Rat Model of Renal Ischemia
- Affiliations
-
- 1Department of Radiology, Konkuk University Medical Center, Research Institute of Medical Science, Konkuk University School of Medicine, Seoul 143-729, Korea. radsijung@kuh.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine; The Institute of Radiation Medicine, Kidney Research Institute; Seoul National University Medical Research Center, Seoul 110-744, Korea.
- 3Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 4Department of Radiology, Seoul National University Boramae Hospital, Seoul 156-707, Korea.
- 5Department of Pathology, Dongguk University International Hospital, Dongguk University College of Medicine, Goyang 410-773, Korea.
- 6Department of Radiology, Cheil General Hospital & Women's Healthcare Center, Kwandong University School of Medicine, Seoul 100-380, Korea.
- KMID: 1779455
- DOI: http://doi.org/10.3348/kjr.2009.10.3.277
Abstract
OBJECTIVE
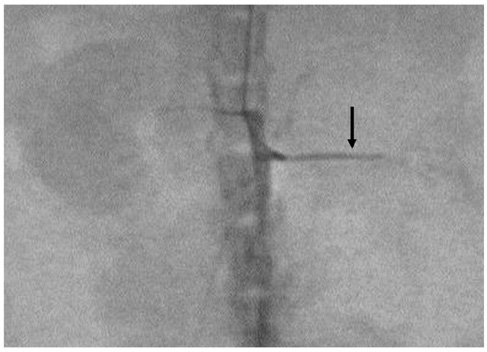
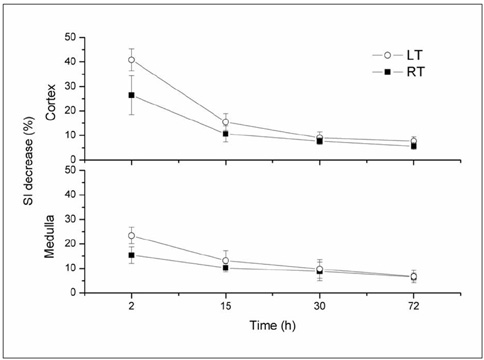
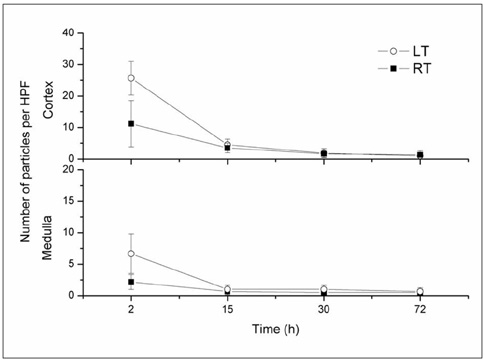
This study was designed to evaluate in vivo MR imaging for the depiction of intraarterially injected superparamagnetic iron oxide (SPIO)-labeled mesenchymal stem cells (MSCs) in an experimental rat model of renal ischemia. MATERIALS AND METHODS: Left renal ischemia was induced in 12 male Sprague-Dawley rats by use of the catheter lodging method. In vivo MR signal intensity variations depicted on T2*-weighted sequences were evaluated in both the left and right kidneys prior to injection (n = 2), two hours (n = 4), 15 hours (n = 2), 30 hours (n = 2) and 72 hours (n = 2) after injection of SPIO-labeled MSCs in both kidneys. Signal intensity variations were correlated with the number of Prussian blue stain-positive cells as visualized in histological specimens. RESULTS: In an in vivo study, it was determined that there was a significant difference in signal intensity variation for both the left and right cortex (40.8 +/- 4.12 and 26.4 +/- 7.92, respectively) and for both the left and right medulla (23.2 +/- 3.32 and 15.2 +/- 3.31, respectively) until two hours after injection (p < 0.05). In addition, signal intensity variation in the left renal cortex was well correlated with the number of Prussian blue stain-positive cells per high power field (r = 0.98, p < 0.05). CONCLUSION: Intraarterial injected SPIO-labeled MSCs in an experimental rat model of renal ischemia can be detected with the use of in vivo MR imaging immediately after injection.
MeSH Terms
Figure
Reference
-
1. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996. 334:1448–1460.2. Shanley PF, Rosen MD, Brezis M, Silva P, Epstein FH, Rosen S. Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am J Pathol. 1986. 122:462–468.3. Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998. 18:490–497.4. Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. Semin Nephrol. 2000. 20:4–19.5. Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004. 22:487–500.6. Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003. 102:3483–3493.7. Krause DS. Plasticity of marrow-derived stem cells. Gene Ther. 2002. 9:754–758.8. Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002. 62:1285–1290.9. Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003. 112:42–49.10. Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003. 228:480–487.11. Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999. 10:186–191.12. Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000. 18:410–414.13. Hauger O, Frost EE, van Heeswijk R, Deminière C, Xue R, Delmas Y, et al. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology. 2006. 238:200–210.14. Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004. 17:484–499.15. Ittrich H, Lange C, Tögel F, Zander AR, Dahnke H, Westenfelder C, et al. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: detection and monitoring at 3T. J Magn Reson Imaging. 2007. 25:1179–1191.16. Kalish H, Arbab AS, Miller BR, Lewis BK, Zywicke HA, Bulte JW, et al. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: relationship between relaxivities, electrostatic forces, and chemical composition. Magn Reson Med. 2003. 50:275–282.17. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005. 289:F31–F42.18. Firbank MJ, Coulthard A, Harrison RM, Williams ED. A comparison of two methods for measuring the signal to noise ratio on MR images. Phys Med Biol. 1999. 44:N261–N264.19. Jo SK, Hu X, Kobayashi H, Lizak M, Miyaji T, Koretsky A, et al. Detection of inflammation following renal ischemia by magnetic resonance imaging. Kidney Int. 2003. 64:43–51.20. Bos C, Delmas Y, Desmoulière A, Solanilla A, Hauger O, Grosset C, et al. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004. 233:781–789.21. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003. 107:2290–2293.22. Bulte JW, Ben-Hur T, Miller BR, Mizrachi-Kol R, Einstein O, Reinhartz E, et al. MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med. 2003. 50:201–205.23. Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001. 19:1141–1147.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo Tracking of Mesenchymal Stem Cells Labeled with a Novel Chitosan-coated Superparamagnetic Iron Oxide Nanoparticles using 3.0T MRI
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Penile Cavernosal Tissues of Streptozotocin-induced Diabetic Rats Using Molecular Magnetic Resonance Imaging
- Serial MR Imaging of Magnetically Labeled Humen Umbilical Vein Endothelial Cells in Acute Renal Failure Rat Model
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Rabbit Bladder Using Molecular MRI
- In Vivo Stem Cell Imaging Principles and Applications