J Korean Med Sci.
2009 Aug;24(4):701-707. 10.3346/jkms.2009.24.4.701.
Preliminary Effects of Oral Uridine on the Ocular Surface in Dry Eye Patients
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine and Seoul National University Hospital Clinical Research Institute, Seoul, Korea. kmk9@snu.ac.kr
- 2Department of Ophthalmology, Kunkuk University Hospital, Seoul, Korea.
- 3MD Bioalpha, Suwon, Korea.
- KMID: 1779207
- DOI: http://doi.org/10.3346/jkms.2009.24.4.701
Abstract
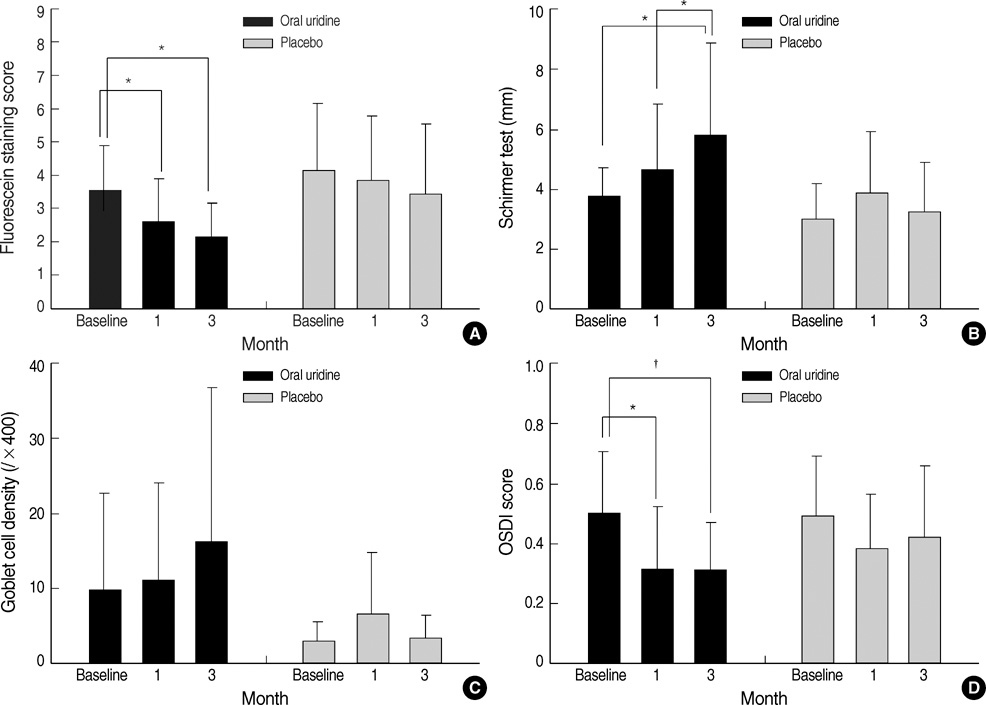
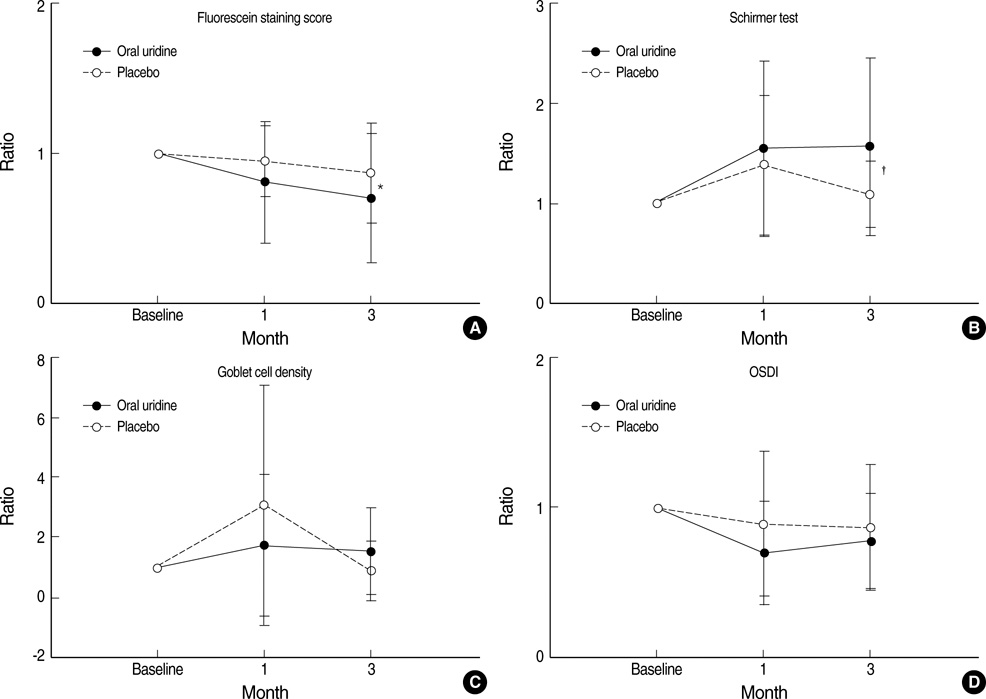
- We designed a randomized, double blinded, 3-months controlled prospective clinical study to investigate effects of oral uridine on the ocular surface in dry eye patients. Twenty-seven patients who diagnosed as dry eye with lower than 5 mm of wetting in the Schirmer strip, with corneal epithelial erosion and who completely followed-up till 3 months were enrolled. Corneal-conjunctival fluorescein staining, non-anesthetic Schirmer test, impression cytology, and Ocular Surface Disease Index (OSDI) were evaluated in the experimental and placebo groups at the baseline, 1 and 3 months after start of medication in a double blinded manner. Fluorescein stain score of the cornea was markedly decreased in oral uridine group compared to the placebo group at 3 months after medication (P=0.032, Mann-Whitney U test). The Schirmer wetting score for the oral uridine group was significantly increased (P=0.001, Wilcoxon signed rank test) at 3 months and its difference between two groups was statistically significant (P=0.030, Mann-Whitney U test). OSDI scores were significantly decreased at 1 and 3 months in treatment group. Oral uridine is effective in treatment of dry eyes.
Keyword
MeSH Terms
Figure
Reference
-
1. Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997. 124:723–728.
Article2. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998. 17:584–589.3. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000. 107:631–639.4. Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002. 86:181–184.
Article5. Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001. 42:96–100.6. Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002. 18:363–370.
Article7. Yerxa BR, Mundasad M, Sylvester RN, Garden JC, Cooper M, Kellerman DJ. Ocular safety of INS365 ophthalmic solution, a P2Y2 agonist, in patients with mild to moderate dry eye disease. Adv Exp Med Biol. 2002. 506(Pt B):1251–1257.8. Tauber J, Davitt WF, Bokosky JE, Nichols KK, Yerxa BR, Schaberg AE, LaVange LM, Mills-Wilson MC, Kellerman DJ. Double-blinded, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea. 2004. 23:784–792.9. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998. 50:413–492.10. Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998. 67:341–346.11. Oh JY, In YS, Kim MK, Ko JH, Lee HJ, Shin KC, Lee SM, Wee WR, Lee JH, Park M. Protective effect of uridine on cornea in a rabbit dry eye model. Invest Ophthalmol Vis Sci. 2007. 48:1102–1109.
Article12. Pitsillides AA, Wilkinson LS, Mehdizadeh S, Bayliss MT, Edwards JC. Uridine diphosphoglucose dehydrogenase activity in normal and rheumatoid synovium: the description of a specialized synovial lining cell. Int J Exp Pathol. 1993. 74:27–34.13. Magee C, Nurminskaya M, Linsenmayer TF. UDP-glucose pyrophosphorylase: up-regulation in hypertrophic cartilage and role in hyaluronan synthesis. Biochem J. 2001. 360:667–674.
Article14. Pflugfelder SC, Geerling G, Kinoshita S. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007. 5:163–178.15. Wilson SE, Stulting RD. Agreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea. 2007. 26:284–289.
Article16. Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O'Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC. Dysfunctional Tear Syndrome Study Group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006. 25:900–907.17. Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol. 2004. 15:299–304.
Article18. Norn MS. Lissamine green. Vital staining of cornea and conjunctiva. Acta Ophthalmol (Copenh). 1973. 51:483–491.19. Martinez AJ, Mills MB, Jaceldo KB, Tio FO, Aigbivbalu IB, Hilsenbeck SB, Yee RW. Standardization of conjunctival impression cytology. Cornea. 1995. 14:515–522.
Article20. Clinch TE, Benedetto DA, Felberg NT, Laibson PR. Schirmer's test: a closer look. Arch Ophthalmol. 1983. 101:1383–1386.21. Moses RA, Parkison G, Schuchardt R. A standard large wound of the corneal epithelium in the rabbit. Invest Ophthalmol Vis Sci. 1979. 18:103–106.22. Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977. 16:597–613.23. Olsen EG, Davanger M. The healing of rabbit corneal endothelium. Acta Ophthalmol (Copenh). 1984. 62:796–807.
Article24. Li Y, Kuang K, Yerxa B, Wen Q, Rosskothen H, Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2 receptor agonists stimulate Cl- and fluid secretion. Am J Physiol Cell Physiol. 2001. 281:C595–C602.25. Murakami T, Fujihara T, Nakamura M, Nakata K. P2Y(2) receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000. 21:782–787.
Article26. Srinivas SP, Yeh JC, Ong A, Bonanno JA. Ca2+ mobilization in bovine corneal endothelial cells by P2 purinergic receptors. Curr Eye Res. 1998. 17:994–1004.
Article27. Pediani JD, McGrath JC, Wilson SM. P2Y receptor-mediated Ca2+ signalling in cultured rat aortic smooth muscle cells. Br J Pharmacol. 1999. 126:1660–1666.28. Clarke LL, Harline MC, Otero MA, Glover GG, Gerrad RC, Krugh B, Walker NM, Gonzalez FA, Turner JT, Weisman GA. Desensitization of P2Y2 receptor-activated transepithelial anion secretion. Am J Physiol. 1999. 276:C777–C787.29. Walker UA, Venhoff N. Uridine in the prevention and treatment of NRTI-related mitochondrial toxicity. Antivir Ther. 2005. 10:Suppl 2. M117–M123.30. Kunzelmann K, Mall M. Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics. Am J Respir Med. 2003. 2:299–309.31. Girot R, Hamet M, Perignon JL, Guesnu M, Fox RM, Cartier P, Durandy A, Griscelli C. Cellular immune deficiency in two siblings with hereditary orotic aciduria. N Engl J Med. 1983. 308:700–704.
Article32. Van Groeningen CJ, Peters GJ, Nadal JC, Laurensse E, Pinedo HM. Clinical and pharmacologic study of orally administered uridine. J Natl Cancer Inst. 1991. 83:437–441.33. Al Safarjalani ON, Zhou XJ, Naguib FN, Shi J, Schinazi RF, el Kouni MH. Enhancement of the bioavailability of oral uridine by coadministration of 5-(phenylthio)acyclouridine, a uridine phosphorylase inhibitor: implications for uridine rescue regimens in chemotherapy. Cancer Chemother Pharmacol. 2001. 48:389–397.
Article34. Venhoff N, Zilly M, Lebrecht D, Schirmer D, Klinker H, Thoden J, Langmann P, Walker UA. Uridine pharmacokinetics of mitocnol, a sugar cane extract. AIDS. 2005. 19:739–740.
Article35. Tsubota K, Kaido M, Yagi Y, Fujihara T, Shimmura S. Diseases associated with ocular surface abnormalities: the importance of reflex tearing. Br J Ophthalmol. 1999. 83:89–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent treatment of dry eye
- Effects of Carbomer Eye Gels on the Ocular Surface in Dry Eye Patients
- The pathogenesis of dry eye disease and trends in treatment
- Pharmacokinetics of Uridine Following Ocular, Oral and Intravenous Administration in Rabbits
- Combined Effect of Rebamipide 2% and Hyaluronic Acid 0.15% on Dry Eye after Cataract Surgery