J Korean Med Sci.
2009 Jun;24(3):403-412. 10.3346/jkms.2009.24.3.403.
Effects of Mixed Herbal Extracts from Parched Puerariae Radix, Gingered Magnoliae Cortex, Glycyrrhizae Radix and Euphorbiae Radix (KIOM-79) on Cardiac Ion Channels and Action Potentials
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul, Korea. sjoonkim@snu.ac.kr
- 2Department of Herbal Pharmaceutical Development, Korea Institute of Oriental Medicine, Daejeon, Korea.
- 3National Research Laboratory for Mitochondrial Signaling, Department of Physiology and Biophysics, Cardiovascular and Metabolic Disease Center, Inje University College of Medicine, Busan, Korea.
- 4Department of Physiology and Research Institute for Biomacromolecules, University of Ulsan College of Medicine, Seoul, Korea.
- 5Kidney Research Institute (KRI), Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 1779155
- DOI: http://doi.org/10.3346/jkms.2009.24.3.403
Abstract
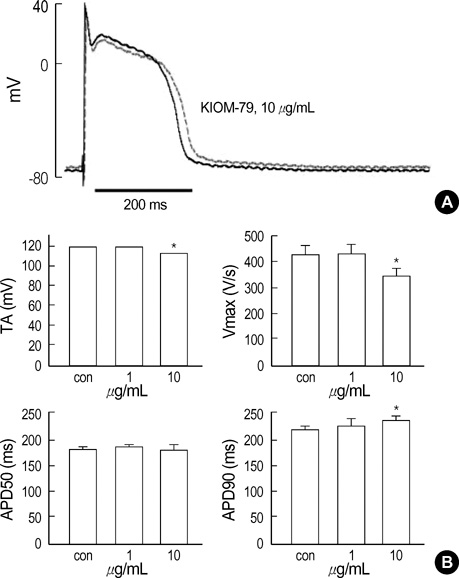
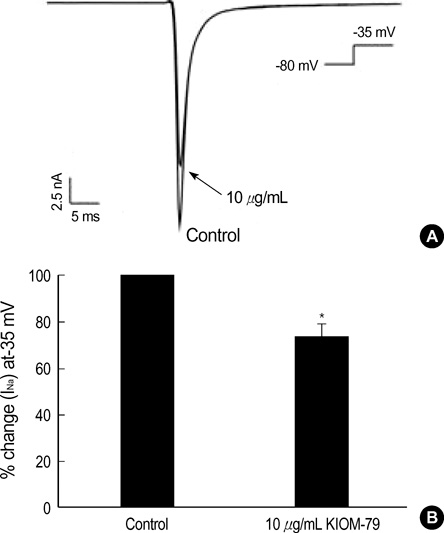
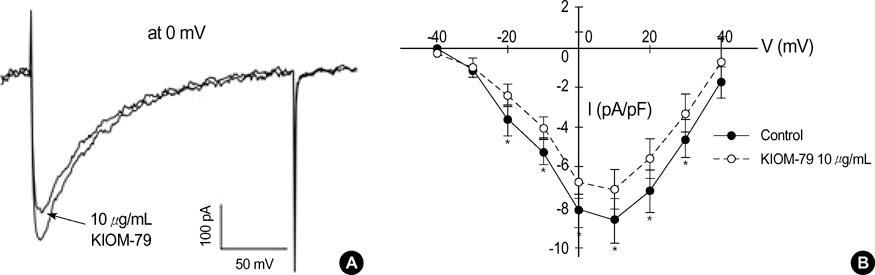
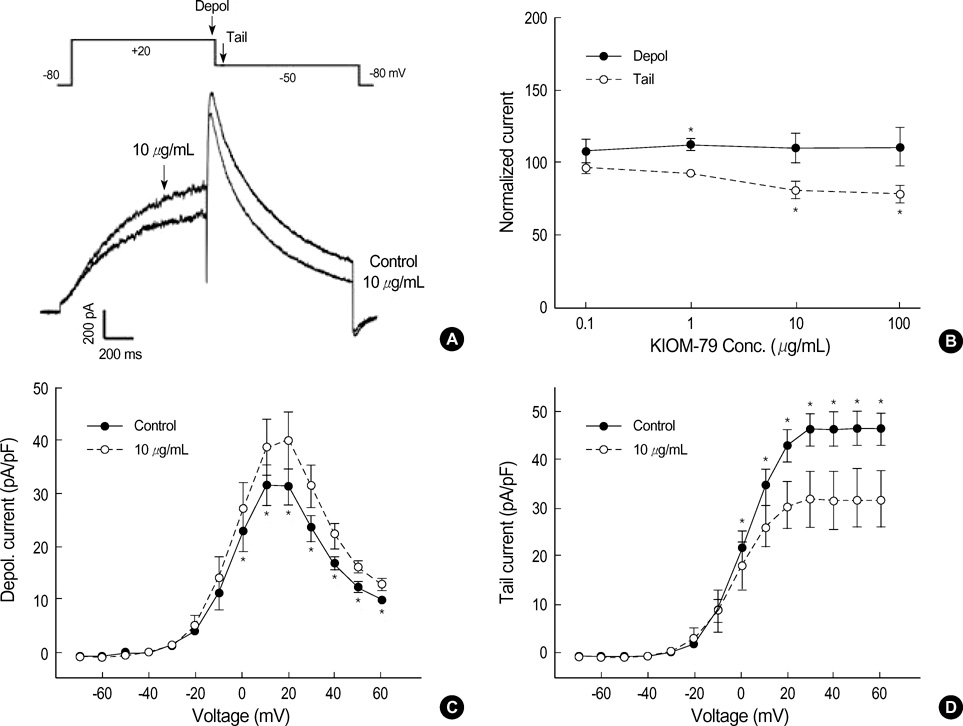
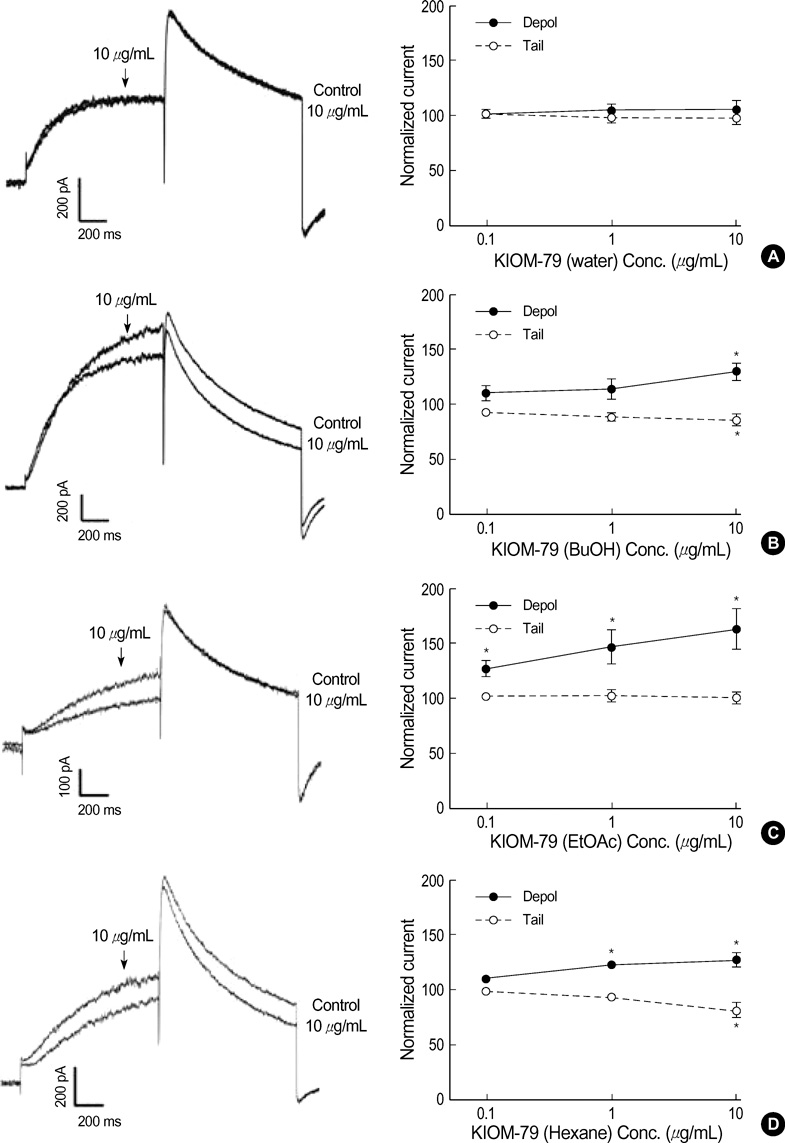
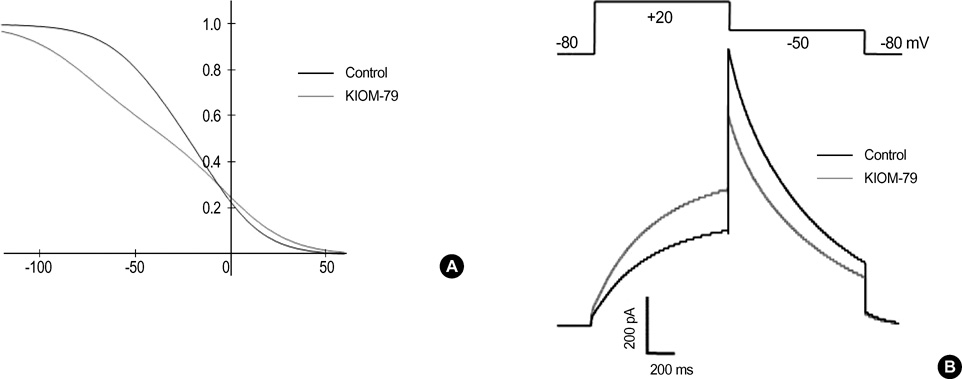
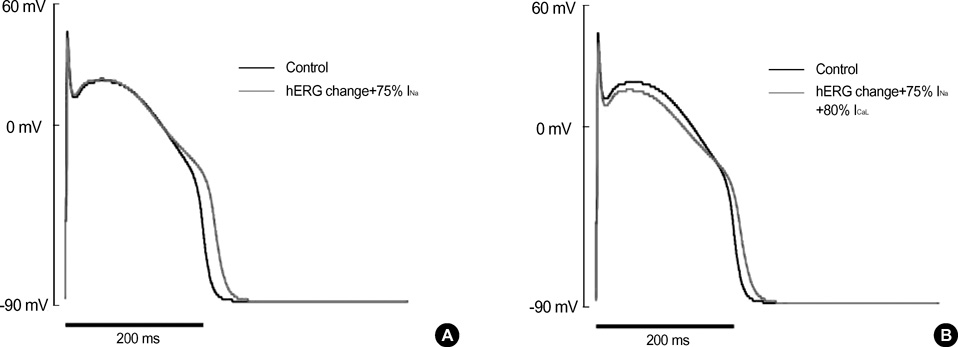
- KIOM-79, a mixture of ethanol extracts from four herbs (parched Puerariae radix, gingered Magnoliae cortex, Glycyrrhizae radix and Euphorbiae radix), has been developed for the potential therapeutic application to diabetic symptoms. Because screening of unexpected cardiac arrhythmia is compulsory for the new drug development, we investigated the effects of KIOM-79 on the action potential (AP) and various ion channel currents in cardiac myocytes. KIOM-79 decreased the upstroke velocity (Vmax) and plateau potential while slightly increased the duration of action potential (APD). Consistent with the decreased Vmax and plateau potential, the peak amplitude of Na+ current (INa) and Ca2+ current (ICa,L) were decreased by KIOM-79. KIOM-79 showed dual effects on hERG K+ current; increase of depolarization phase current (Idepol) and decreased tail current at repolarization phase (Itail). The increase of APD was suspected due to the decreased Itail. In computer simulation, the change of cardiac action potential could be well simulated based on the effects of KIOM-79 on various membrane currents. As a whole, the influence of KIOM-79 on cardiac ion channels are minor at concentrations effective for the diabetic models (0.1-10 microg/mL). The results suggest safety in terms of the risk of cardiac arrhythmia. Also, our study demonstrates the usefulness of the cardiac computer simulation in screening drug-induced long-QT syndrome.
Keyword
MeSH Terms
-
Action Potentials/*drug effects
Animals
Cell Line
Computer Simulation
Female
Ginger/chemistry
Humans
Ion Channels/*physiology
Long QT Syndrome/diagnosis
Male
Membrane Potentials/drug effects/physiology
Myocytes, Cardiac/*drug effects/physiology
Patch-Clamp Techniques
Plant Extracts/*pharmacology
Pueraria/chemistry
Purkinje Fibers/drug effects/physiology
Rabbits
Rats
Rats, Sprague-Dawley
Figure
Reference
-
1. Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004. 92:1–21.
Article2. Aida K, Tawata M, Shindo H, Onaya T, Sasaki T, Yamaguchi T, Chin M, Mitsuhashi H. Isoliquiritigenin: a new aldose reductase inhibitor from glycyrrhizae radix. Planta Medicine. 1990. 56:254–258.
Article3. Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethnopharmacol. 1998. 61:101–110.
Article4. Hur J. Donguibogam Parallel Version. Committee of Dongui Bogam Translation. 1999. Seoul, Korea: Bupin Publishes Co..5. Kang KA, Chae S, Koh YS, Kim JS, Lee JH, You HJ, Hyun JW. Protective effect of puerariae radix on oxidative stress induced by hydrogen peroxide and streptozotocin. Biol Pharm Bull. 2005. 28:1154–1160.
Article6. Kim CS, Sohn EJ, Kim YS, Jung DH, Jang DS, Lee YM, Kim DH, Kim JS. Effects of KIOM-79 on hyperglycemia and diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. J Ethnopharmacol. 2007. 111:240–247.
Article7. Jeon YJ, Li MH, Lee KY, Kim JS, You HJ, Lee SK, Sohn HM, Choi SJ, Koh JW, Chang IY. KIOM-79 inhibits LPS-induced iNOS gene expression by blocking NF-kappaB/Rel and p38 kinase activation in murine macrophages. J Ethnopharmacol. 2006. 108:38–45.8. Kim YS, Jung DH, Kim NH, Lee YM, Jang DS, Song GY, Kim JS. KIOM-79 inhibits high glucose or AGEs-induced VEGF expression in human retinal pigment epithelial cells. J Ethnopharmacol. 2007. 112:166–172.
Article9. Netzer R, Ebneth A, Bischoff U, Pongs O. Screening lead compounds for QT interval prolongation. Drug Discov Today. 2001. 6:78–84.
Article10. Marban E. Cardiac channelopathies. Nature. 2002. 415:213–218.
Article11. Roden DM. Clinical practice. Long QT syndrome. New Engl J Med. 2008. 358:169–176.12. Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002. 90:939–950.
Article13. Cheng JH, Kodama I. Two components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarization. Acta Pharmacol Sin. 2004. 25:137–145.14. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006. 440:463–469.
Article15. Yuill KH, Convery MK, Dooley PC, Doggrell SA, Hancox JC. Effects of BDF 9198 on action potentials and ionic currents from guinea-pig isolated ventricular myocytes. Br J Pharmacol. 2000. 130:1753–1766.
Article16. Yoon JY, Ahn SH, Oh H, Kim YS, Ryu SY, Ho WK, Lee SH. A novel Na+ channel agonist, dimethyl lithospermate B, slows Na+ current inactivation and increases action potential duration in isolated rat ventricular myocytes. Br J Pharmacol. 2004. 143:765–773.17. Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of hERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998. 74:230–241.
Article18. Hille B. Ion Channels of Excitable Membranes. 2001. 3rd Ed. Sunderland, MA: Sinauer Associates.19. Woo WS. Methods in Natural Product Chemistry. 2002. Seoul: Seoul National University Press;12–13.20. Zhang Y, Chen J, Zhang C, Wu W, Liang X. Analysis of the estrogenic components in kudzu root by bioassay and high performance liquid chromatography. J Steroid Biochem Mol Biol. 2005. 94:375–381.
Article21. Otsuka H. Sarker SD, Latif Z, Gray AI, editors. Purification by Solvent Extraction Using Partition Coefficient. Methods in Biotechnology. 2005. Vol. 20 Natural Products Isolation:2nd Ed. Totowa, NJ: Humana Press;269–273.
Article22. Matsuoka S, Sarai N, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 2003. 53:105–123.
Article23. Sarai N, Matsuoka S, Noma A. simBio: a Java package for the development of detailed cell models. Prog Biophys Mol Biol. 2006. 90:360–377.
Article24. Meyer T, Sartipy P, Blind F, Leisgen C, Guenther E. New cell models and assays in cardiac safety profiling. Expert Opin Drug Metab Toxicol. 2007. 3:507–517.
Article25. Qian Y, Li Z, Huang L, Han X, Sun J, Zhou H, Liu Z. Blocking effect of puerarin on calcium channel in isolated guinea pig ventricular myocytes. Chin Med J (Engl). 1999. 112:787–789.26. Zhang GQ, Hao XM, Dai DZ, Fu Y, Zhou PA, Wu CH. Puerarin blocks Na+ current in rat ventricular myocytes. Acta Pharmacol Sin. 2003. 24:1212–1216.27. Lin YR, Chen HH, Ko CH, Chan MH. Differential inhibitory effects of honokiol and magnolol on excitatory amino acid-evoked cation signals and NMDA-induced seizures. Neuropharmacology. 2005. 49:542–550.
Article28. Liu YC, Lo YC, Huang CW, Wu SN. Inhibitory action of ICI-182, 780, an estrogen receptor antagonist, on BK(Ca) channel activity in cultured endothelial cells of human coronary artery. Biochem Pharmacol. 2003. 66:2053–2063.29. Wu SN, Chen CC, Li HF, Lo YK, Chen SA, Chiang HT. Stimulation of the BK(Ca) channel in cultured smooth muscle cells of human trachea by magnolol. Thorax. 2002. 57:67–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Supplementation of Puerariae Radix Ethanol Extract on the Antioxidative Defense System in Rats
- Effect of Radix Puerariae on Alcohol Craving and Regional Cerebral Blood Flow in the Patients with Alcohol Dependence
- Radix augmentation using temporalis fascia graft
- Effects of Polygalae Radix on Apomorphine-Induced Stereotyped Behaviors in Mice
- Promoting Effect of a Mixture of 8 Herbal Extracts (SPELA 707) on Hair Growth