J Korean Med Sci.
2009 Jun;24(3):384-391. 10.3346/jkms.2009.24.3.384.
Ca2+-activated K+ Current in Freshly Isolated c-Kit Positive Cells in Guinea-pig Stomach
- Affiliations
-
- 1Department of Physiology, Chungbuk National University, College of Medicine, Cheongju, Korea. physiokyc@chungbuk.ac.kr
- 2Department of Physiology, Nagoya City University Medical School, Nagoya, Japan.
- 3Department of Physiology, College of Medicine, Shanghai Jiaotong University, Shanghai, P.R. China.
- 4Department of Pharmacology, Chungbuk National University, College of Medicine, Cheongju, Korea.
- 5Department of Pathology, Samsung Medical Center, SungKyunKwan University, Seoul, Korea.
- KMID: 1779152
- DOI: http://doi.org/10.3346/jkms.2009.24.3.384
Abstract
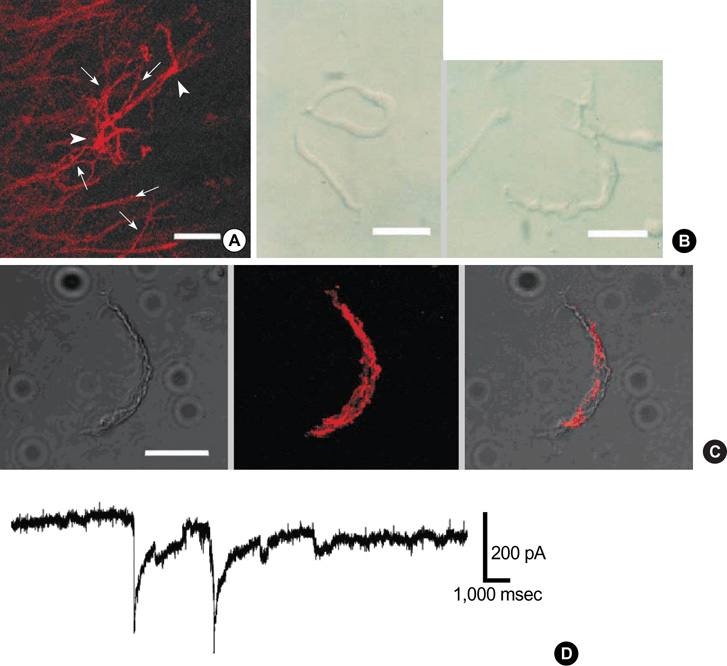
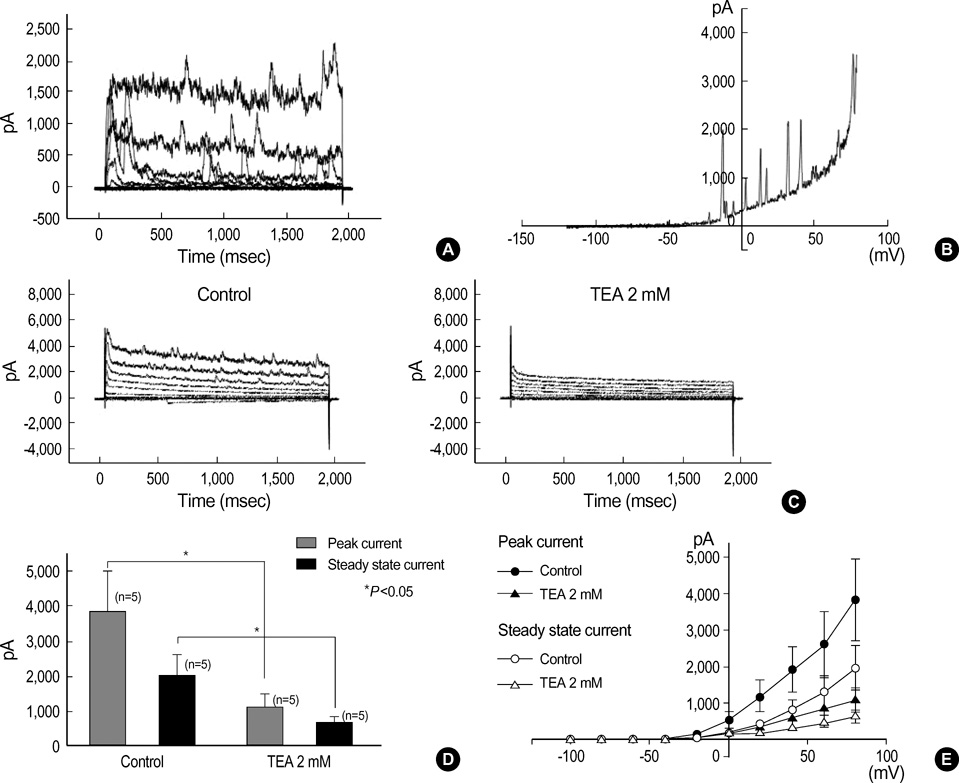
- This study was designed to isolate Ca2+-activated K+ current (IKCa) and elucidate its physiological significance in freshly isolated interstitial cells of Cajal (ICCs) of guinea-pig stomach. Single ICC was freshly isolated by enzymatically dissociating from myenteric border of gastric antrum free of circular muscles, and conventional whole-cell voltage clamp technique including immunohistochemical techniques were employed to characterize the cells: In myenteric border of gastric antrum, ICC-MY (ICCs from myenteric border) were detected by immunohistochemical reactivity, and single ICC-MY which has many branches was immunohistochemically c-Kit positive. Under K+-rich and 0.1 mM ethylene glycol-bis (2-aminoethyl ether)-N,N,N',N'-tetraacetic acid pipette solution, ICC produced spontaneous inward current (-256+/-92.2 pA). When step-depolarizing pulse from -80 to +80 mV was applied at holding potential (Vh) of -80 mV, voltage-dependent outward currents were recorded with superimposed spontaneous transient outward currents (STOCs). Both STOCs and outward currents were reversibly affected by tetraethylammonium chloride (TEA) and iberiotoxin (IbTX); 2 mM TEA and 200 nM IbTX completely abolished STOCs and significantly inhibited outward K+ current over the whole potential range tested for current/voltage (I/V) relationship. In addition, TEA delayed repolarization phase of spontaneous inward current. The present results indicate the presence of IKCa in a single ICC, and it might be involved in regulation of repolarizing phase of spontaneous inward current in guinea-pig stomach.
Keyword
MeSH Terms
Figure
Reference
-
1. Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/Kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995. 373:347–349.
Article2. Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the protooncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994. 480:91–97.
Article3. Chen H, Redelman D, Ro S, Ward SM, Ördög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007. 292:C497–C507.
Article4. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2005. 68:307–343.
Article5. Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999. 514:515–531.
Article6. Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000. 525:105–111.
Article7. Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Aca Sci USA. 1996. 93:12008–12013.
Article8. Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998. 115:314–329.
Article9. Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006. 573:147–159.
Article10. Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998. 513:203–213.
Article11. Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA. 1989. 86:7280–7284.
Article12. Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998. 4:848–851.
Article13. Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002. 123:1627–1636.
Article14. Koh SD, Jun JY, Kim TW, Sanders KM. A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002. 540:803–814.15. Zhu Y, Golden CM, Ye J, Wang XY, Akbarali HI, Huizinga JD. ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G1249–G1258.16. Ward SM, Ördög T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000. 525:355–361.
Article17. Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004. 117:2813–2825.18. Nakayama S, Ohya S, Liu HN, Watanabe T, Fruzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005. 118:4163–4173.19. Fujii K, Inoue R, Yamanaka K, Yoshitomi T. Effects of calcium antagonists on smooth muscle membranes of the canine stomach. Gen Pharmacol. 1985. 16:217–221.
Article20. Sanders KM, Publicover NG. Chultz S, Rauner BB, Wood JD, editors. Electrophysiology of gastric musculature. Handbook of Physiology: the Gastrointestinal System. 1989. Bethesda, MD: American Physiology Society;187–216.21. Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986. 371:45–67.
Article22. Carl A, Sanders KM. Ca2+-activated K+ channels of canine colonic myocytes. Am J Physiol. 1989. 257:C470–C480.23. Carl A, Frey BW, Ward SM, Sanders KM, Kenyon JL. Inhibition of slow-wave repolarization and Ca2+-activated K+ channels by quaternary ammonium ions. Am J Physiol. 1993. 264:C625–C631.24. Szurszewski JH. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978. 277:91–102.
Article25. Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004. 559:411–422.
Article26. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.27. Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002. 541:797–810.28. Lee HK, Sanders KM. Comparision of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993. 460:135–152.29. Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997. 273:G1233–G1245.
Article30. Wang XY, Vannucchi MG, Nieuwmeyer F, Ye J, Faussone-Pellegrini MS, Huizinga JD. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005. 167:437–453.
Article31. Carl A, McHale NG, Publicover NG, Sanders KM. Participation of Ca2+-activated K+ channels in electrical activity of canine gastric smooth muscle. J Physiol. 1990. 429:205–221.32. Ohya Y, Kitamura K, Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987. 152:C401–C410.
Article33. Singer JJ, Walsh JV Jr. Characterization of calcium activated potassium channels in single smooth muscle cells using patch-clamp technique. Pflügers Arch. 1987. 408:98–111.34. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol. 2005. 288:C1255–C1268.35. Benham CD, Bolton TB, Lang RJ, Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch. 1985. 403:120–127.36. Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996. 20:141–152.
Article37. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995. 270:633–637.
Article38. ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV Jr. Ca2+ sparks activated K+ and Cl- channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol. 1998. 513:711–718.39. Ganitkevich VY, Isenberg G. Contribution of Ca2+ induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea pig urinary bladder. J Physiol. 1992. 458:119–137.40. Iino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 1990. 54:345–354.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Voltage-dependent Ca2+ Current Identified in Freshly Isolated Interstitial Cells of Cajal (ICC) of Guinea-pig Stomach
- Ca2 -activated K Currents of Pancreatic Duct Cells in Guinea-pig
- Effect of Kanamycin on Calcium Current of Rabbit Ventricular Myocyte
- Depolarizing Mechanisms of Acetylcholine in Gastric Smooth Muscle
- Effects of Vanadate on Cellular Ca2+ Movements in Guinea Pig Papillary Muscles