J Korean Med Sci.
2012 Oct;27(10):1170-1176. 10.3346/jkms.2012.27.10.1170.
Tissue Responses to Endovascular Stent Grafts for Saccular Abdominal Aortic Aneurysms in a Canine Model
- Affiliations
-
- 1Department of Radiology, National Cancer Center, Goyang, Korea.
- 2Department of Radiology, Seoul National University Boramae Hospital, Seoul, Korea.
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Radiology and Institute of Radiation Medicine, Seoul National University College of Medicine, Clinical Research Institute, Seoul, Korea. chungjw@snu.ac.kr
- KMID: 1778822
- DOI: http://doi.org/10.3346/jkms.2012.27.10.1170
Abstract
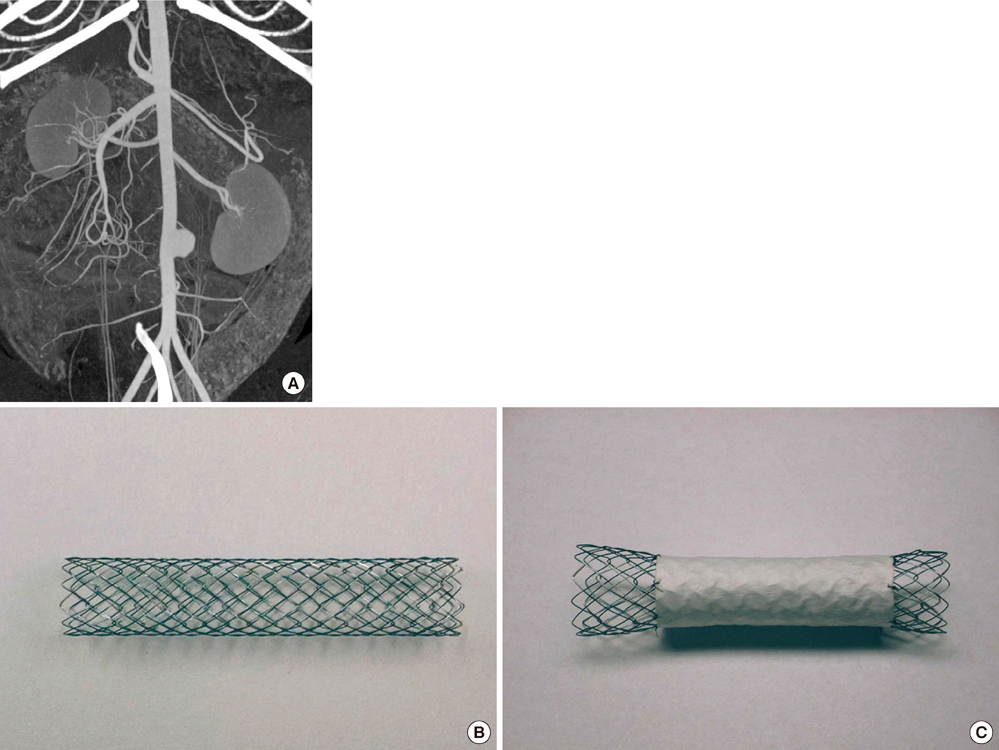
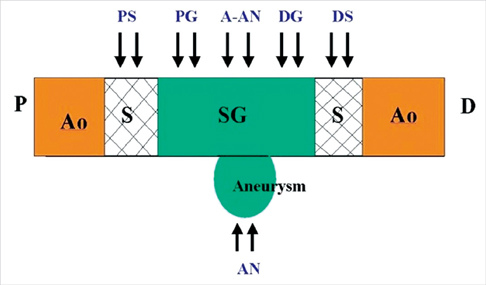
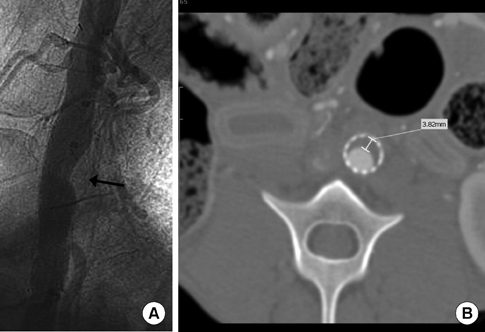
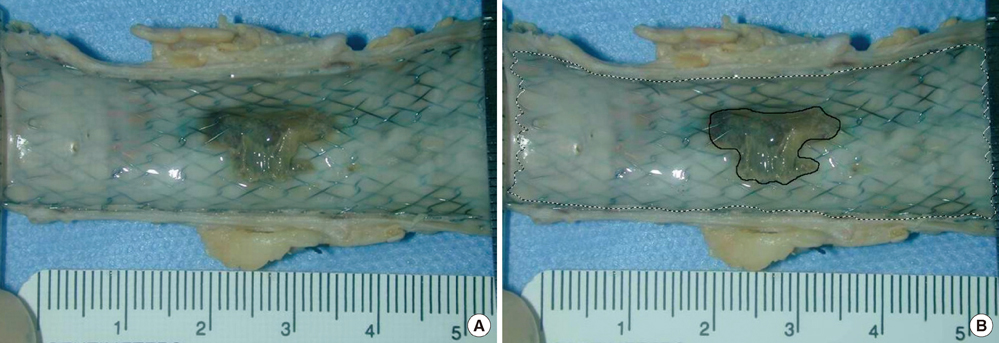
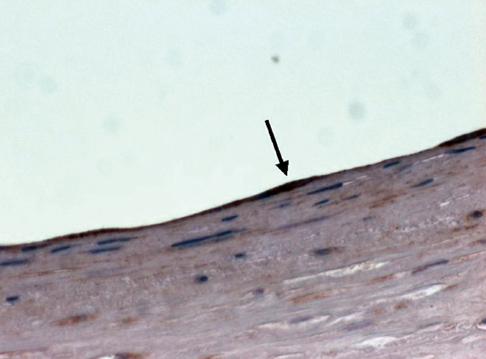
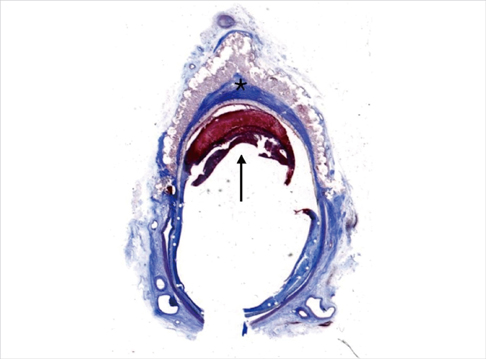
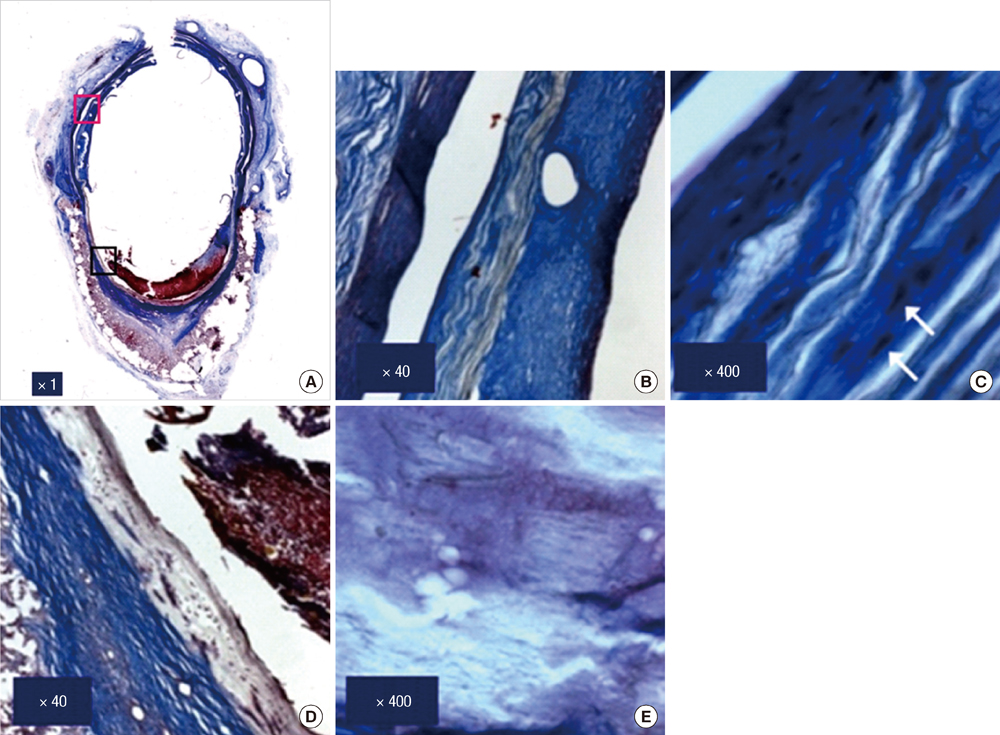
- We investigated tissue responses to endoskeleton stent grafts for saccular abdominal aortic aneurysms (AAAs) in canines. Saccular AAAs were made with Dacron patch in 8 dogs, and were excluded by endoskeleton stent grafts composed of nitinol stent and expanded polytetrafluoroethylene graft. Animals were sacrificed at 2 months (Group 1; n = 3) or 6 months (Group 2; n = 5) after the placement, respectively. The aortas embedding stent grafts were excised en bloc for gross inspection and sliced at 5 to 8 mm intervals for histopathologic evaluation. Stent grafts were patent in all except a dog showing a thrombotic occlusion in Group 2. In the 7 dogs with patent lumen, the graft overhanging the saccular aneurysm was covered by thick or thin thrombi with no endothelial layer, and the graft over the aortic wall was completely covered by neointima with an endothelial layer. Transgraft cell migration was less active at an aneurysm than at adjacent normal aorta. In conclusion, endoskeleton stent grafts over saccular aneurysms show no endothelial coverage and poor transgraft cell migration in a canine model.
Keyword
MeSH Terms
Figure
Reference
-
1. Dotter CT. Transluminally-placed coilspring endarterial tube grafts: Long-term patency in canine popliteal artery. Invest Radiol. 1969. 4:329–332.2. May J. Abdominal aortic aneurysm: endovascular repair. Med J Aust. 2000. 173:340–341.3. Castelli P, Caronno R, Piffaretti G, Tozzi M, Laganà D, Carrafiello G, Cuffari S, Bacuzzi A. Ruptured abdominal aortic aneurysm: endovascular treatment. Abdom Imaging. 2005. 30:263–269.4. Hinchliffe RJ, Braithwaite BD. Ruptured abdominal aortic aneurysm: endovascular repair does not confer any long-term survival advantage over open repair. Vascular. 2007. 15:191–196.5. Mestres G, Zarka ZA, García-Madrid C, Riambau V. Early abdominal aortic endografts: a decade follow-up results. Eur J Vasc Endovasc Surg. 2010. 40:722–728.6. El-Sayed H, Ramlawi B. The current status of endovascular repair of thoracic aortic aneurysms (TEVAR). Methodist Debakey Cardiovasc J. 2011. 7:15–19.7. Veraldi GF, Furlan F, Tasselli S, Tomasi I, Firpo M. Endovascular repair of intrathoracic left subclavian artery aneurysm with stent grafts: report of a case and review of the literature. Chir Ital. 2005. 57:355–359.8. Lammer J. Femoropopliteal artery obstructions: from the balloon to the stent-graft. Cardiovasc Intervent Radiol. 2001. 24:73–83.9. Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999. 213:759–766.10. Zhang Z, Briana S, Douville Y, Zhao H, Gilbert N. Transmural communication at a subcellular level may play a critical role in the fallout based-endothelialization of dacron vascular prostheses in canine. J Biomed Mater Res A. 2007. 81:877–887.11. Poole-Warren LA, Schindhelm K, Graham AR, Slowiaczek PR, Noble KR. Performance of small diameter synthetic vascular prostheses with confluent autologous endothelial cell linings. J Biomed Mater Res. 1996. 30:221–229.12. Guidoin R, Chakfé N, Maurel S, How T, Batt M, Marois M, Gosselin C. Expanded polytetrafluoroethylene arterial prostheses in humans: histopathological study of 298 surgically excised grafts. Biomaterials. 1993. 14:678–693.13. Wu MH, Shi Q, Onuki Y, Kouchi Y, Sauvage LR. Histologic observation of continuity of transmural microvessels between the perigraft vessels and flow surface microostia in a porous vascular prosthesis. Ann Vasc Surg. 1996. 10:11–15.14. Shi Q, Wu MH, Fujita Y, Ishida A, Wijelath ES, Hammond WP, Wechezak AR, Yu C, Storb RF, Sauvage LR. Genetic tracing of arterial graft flow surface endothelialization in allogeneic marrow transplanted dogs. Cardiovasc Surg. 1999. 7:98–105.15. Dolmatch BL, Tio FO, Li XD, Dong YH. Patency and tissue response related to two types of polytetrafluoroethylene-covered stents in the dog. J Vasc Interv Radiol. 1996. 7:641–649.16. Marty B, Leu AJ, Mucciolo A, von Segesser LK. Biologic fixation of polyester- versus polyurethane-covered stents in a porcine model. J Vasc Interv Radiol. 2002. 13:601–607.17. Wilson EP, White RA, Kopchok GE, Donayre CE, de Virgilio C, Geselschap JH, Heilbron M. Deployment and healing of an ePTFE encapsulated stent endograft in the canine aorta. Ann Vasc Surg. 1997. 11:354–358.18. Virmani R, Kolodgie FD, Dake MD, Silver JH, Jones RM, Jenkins M, Gillespie DL. Histopathologic evaluation of an expanded polytetrafluoro-ethylene-nitinol stent endoprosthesis in canine iliofemoral arteries. J Vasc Interv Radiol. 1999. 10:445–456.19. Bashar AH, Kazui T, Terada H, Suzuki K, Washiyama N, Yamashita K, Baba S. Histological changes in canine aorta 1 year after stent-graft implantation: implications for the long-term stability of device anchoring zones. J Endovasc Ther. 2002. 9:320–332.20. Guidoin R, Douville Y, Baslé MF, King M, Marinov GR, Traoré A, Zhang Z, Guillemot F, Dionne G, Sumanasinghe R, et al. Biocompatibility studies of the Anaconda stent-graft and observations of nitinol corrosion resistance. J Endovasc Ther. 2004. 11:385–403.21. Palmaz JC, Tio FO, Laborde JC, Clem M, Rivera FJ, Murphy KD, Encarnacion CE. Use of stents covered with polytetrafluoroethylene in experimental abdominal aortic aneurysm. J Vasc Interv Radiol. 1995. 6:879–885.22. Benson AE, Palmaz JC, Tio FO, Sprague EA, Encarnacion CE, Josephs SC. Polytetrafluoroethylene-encapsulated stent-grafts: use in experimental abdominal aortic aneurysm. J Vasc Interv Radiol. 1999. 10:605–612.23. Little D, Said JW, Siegel RJ, Fealy M, Fishbein MC. Endothelial cell markers in vascular neoplasms: an immunohistochemical study comparing factor VIII-related antigen, blood group specific antigens, 6-keto-PGF1 alpha, and Ulex europaeus 1 lectin. J Pathol. 1986. 149:89–95.24. Hanel KC, McCabe C, Abbott WM, Fallon J, Megerman J. Current PTFE grafts: a biomechanical, scanning electron, and light microscopic evaluation. Ann Surg. 1982. 195:456–463.25. Ombrellaro MP, Stevens SL, Kerstetter K, Freeman MB, Goldman MH. Healing characteristics of intraarterial stented grafts: effect of intraluminal position on prosthetic graft healing. Surgery. 1996. 120:60–70.26. Ombrellaro MP, Stevens SL, Sciarrotta J, Schaeffer D, Freeman MB, Goldman MH. Effect of intra-arterial environment on endothelialization and basement membrane organization in polytetrafluoroethylene grafts. Am J Surg. 1997. 174:29–32.27. White R, Kopchok G, Zalewski M, Ayres B, Wilson E, de Virgilio C, Donayre C. Comparison of the deployment and healing of thin-walled expanded PTFE stented grafts and covered stents. Ann Vasc Surg. 1996. 10:336–346.28. Dolmatch BL, Dong YH, Trerotola SO, Hunter DW, Brennecke LH, LaBounty R. Tissue response to covered Wallstents. J Vasc Interv Radiol. 1998. 9:471–478.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tissue Responses to Stent Grafts with Endo-Exo-Skeleton for Saccular Abdominal Aortic Aneurysms in a Canine Model
- Simultaneous Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm Combined with Saccular Thoracic Aortic Aneurysm
- Endovascular Stent Graft Repair of Multiple Tuberculous Thoracoabdominal Aneurysms
- Transluminal Endovascular Stent-Graft for the Treatment of Aortic Aneuryms
- Endovascular Treatment of Abdominal Aortic Aneurysm