J Korean Med Sci.
2006 Jun;21(3):397-405. 10.3346/jkms.2006.21.3.397.
Prognostic Evaluation of Nodal Diffuse Large B Cell Lymphoma by Immunohistochemical Profiles with Emphasis on CD138 Expression as a Poor Prognostic Factor
- Affiliations
-
- 1Department of Pathology, College of Medicine and Institute of Biomedical Science, Hanyang University, Seoul, Korea. parkcg@hanyang.ac.kr
- KMID: 1778418
- DOI: http://doi.org/10.3346/jkms.2006.21.3.397
Abstract
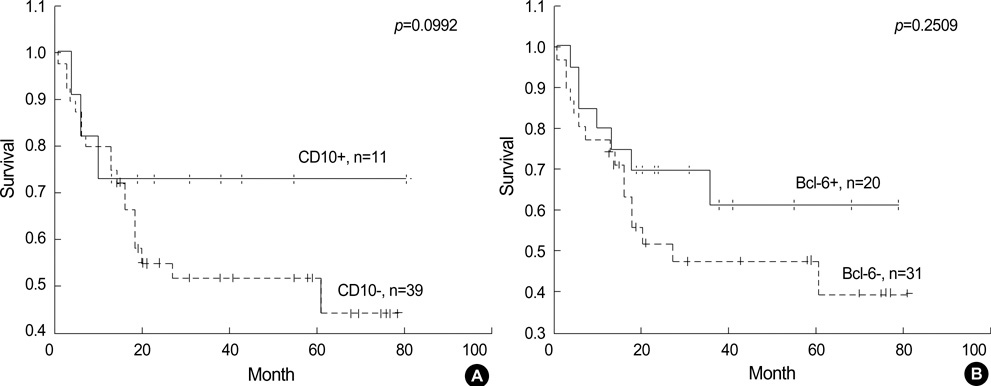
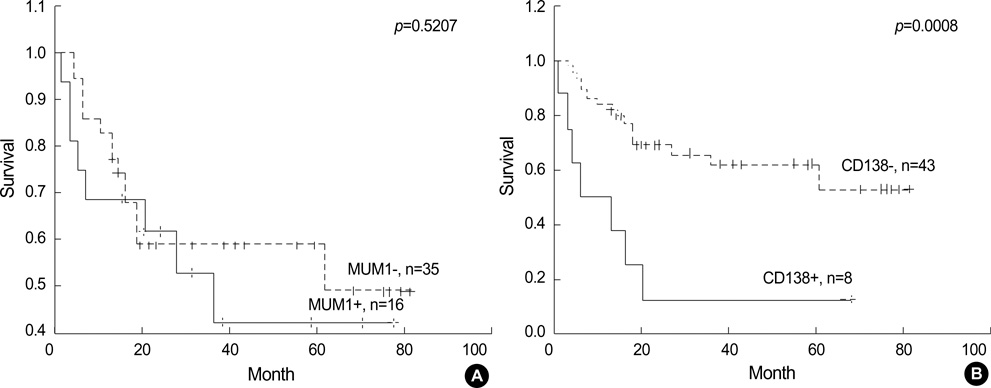
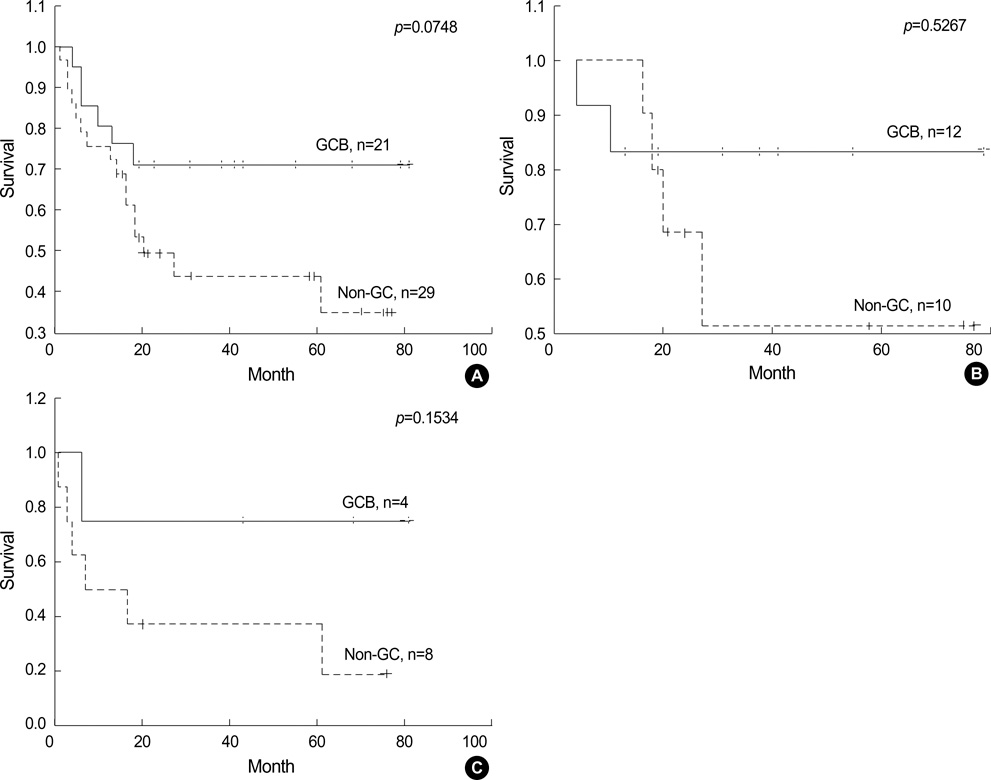
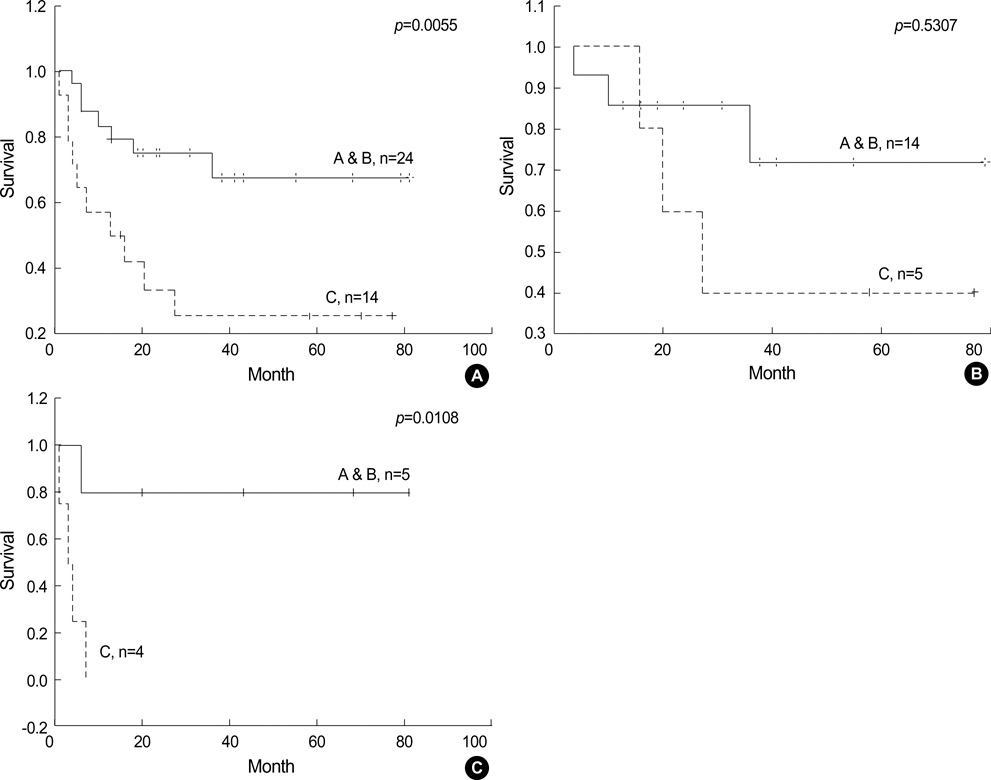
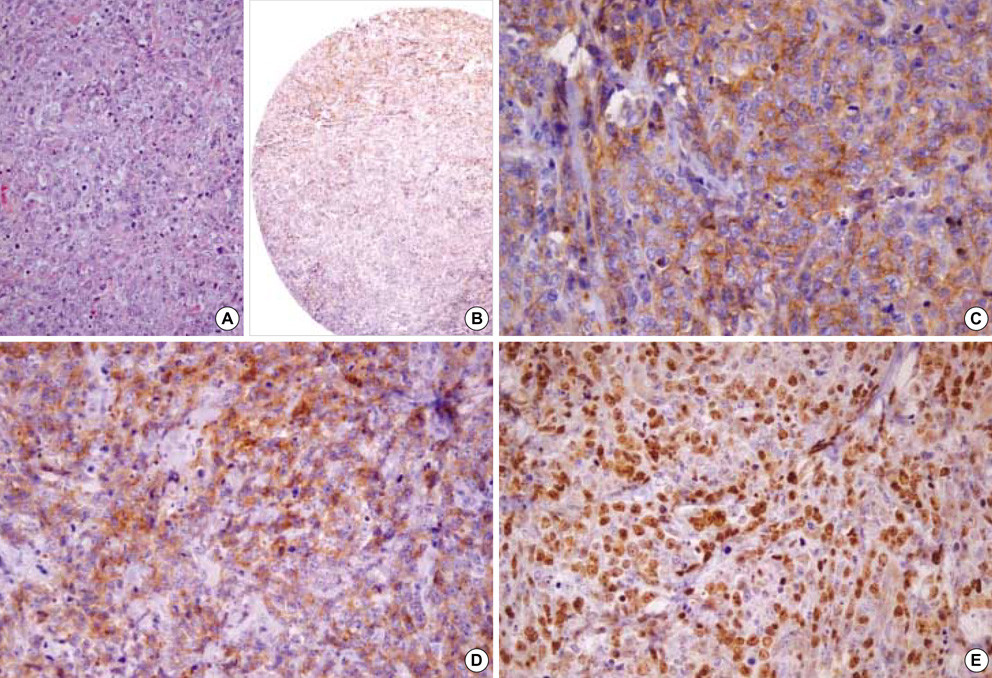
- Recently diffuse large B cell lymphoma (DLBCLs) was reported to be subdivided into germinal center B-cell-like (GCB) and activated B-cell-like (ABC) subgroups by using cDNA microarray and immunohistochemical markers. Tissue microarray blocks were created from 51 nodal DLBCLs with control tissue. Immunohistochemical staining for the above markers were performed. The median follow-up period was 26 months. Nodal DLBCLs were subclassified into GCB [CD10+ or CD10-/Bcl-6+/MUM1-, n=17 (33%)] and non-GC subgroups [CD10-/Bcl-6- or CD10-/Bcl-6+/MUM1+, n=35 (67%)], and were alternatively subclassified into pattern A [+ for GCB marker only, n=12 (23%)], B [Co-positive for both markers, n=13 (33%)], C [+ for activation marker only, n=18 (35%)], and D [- for both markers, n=9 (17%)]. Upon survival analysis, the GCB groups showed a relatively better survival than non-GC groups (p=0.0748). Also, pattern C (p=0.0055) and CD138+ (p=0.0008) patients had significantly lower survival rates. By multivariate analysis, CD138 expression alone was considered as an independent risk factor (p=0.031). In summary, our results add to the registration of prognostic implications for previously reported DLBCL subgroups. CD138 may play an important role as a poor prognostic marker. By using immunohistochemistry, a prognostically important subclassification of DLBCLs is possible.
Keyword
MeSH Terms
-
Tumor Markers, Biological/metabolism
Syndecans/metabolism
Syndecan-1/*biosynthesis
Prognosis
Neprilysin/biosynthesis
Middle Aged
Male
Lymphoma, Large-Cell, Diffuse/*diagnosis/*metabolism/pathology
Lymphoma, B-Cell/*diagnosis/*metabolism/pathology
Humans
*Gene Expression Regulation, Neoplastic
Female
Aged, 80 and over
Aged
Adult
Figure
Reference
-
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997. 89:3909–3918.2. Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001. 13:325–334.
Article3. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000. 403:503–511.
Article4. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, Le-Blanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. Lymphoma/Leukemia Molecular Profiling Project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:1937–1947.
Article5. Barrans SL, Carter I, Owen RG, Davies FE, Patmore RD, Haynes AP, Morgan GJ, Jack AS. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood. 2002. 99:1136–1143.
Article6. Colomo L, Lopez-Guillermo A, Perales M, Rives S, Martinez A, Bosch F, Colomer D, Falini B, Montserrat E, Campo E. Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood. 2003. 101:78–84.
Article7. Linderoth J, Jerkeman M, Cavallin-Stahl E, Kvaloy S, Torlakovic E. Nordic Lymphoma Group Study. Immunohistochemical expression of CD23 and CD40 may identify prognostically favorable subgroups of diffuse large B-cell lymphoma: a Nordic Lymphoma Group Study. Clin Cancer Res. 2003. 9:722–728.8. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004. 103:275–282.9. Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B, Kajdacsy-Balla A, Perkins SL. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004. 28:464–470.
Article10. Zettl A, Meister S, Katzenberger T, Kalla J, Ott MM, Muller-Hermelink HK, Ott G. Immunohistochemical analysis of B-cell lymphoma using tissue microarrays identifies particular phenotypic profiles of B-cell lymphomas. Histopathology. 2003. 43:209–219.
Article11. Milanes-Yearsley M, Hammond ME, Pajak TF, Cooper JS, Chang C, Griffin T, Nelson D, Laramore G, Pilepich M. Tissue micro-array: a cost and time-effective method for correlative studies by regional and national cancer study groups. Mod Pathol. 2002. 15:1366–1373.
Article12. Gatter KC, Warnke RA. Jaffe ES, Harris NL, Stein H, editors. Diffuse large B-cell lymphoma. WHO Classification of Tumors: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. 2001. Lyon, France: IARCPress;171–174.13. Pileri SA, Dirnhofer S, Went P, Ascani S, Sabattini E, Marafioti T, Tzankov A, Leoncini L, Falini B, Zinzani PL. Diffuse large B-cell lymphoma: one or more entities? Present controversies and possible tools for its subclassification. Histopathology. 2002. 41:482–509.
Article14. Fisher RI, Shah P. Current trends in large cell lymphoma. Leukemia. 2003. 17:1948–1960.
Article15. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation; a population-based study of 1575 cases. Br J Haematol. 2004. 124:151–159.16. Lopez-Guillermo A, Colomo L, Jimenez M, Bosch F, Villamor N, Arenillas L, Muntanola A, Montoto S, Gine E, Colomer D, Bea S, Campo E, Montserrat E. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005. 23:2797–2804.17. Dogan A, Bagdi E, Munson P, Isaacson PG. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 2000. 24:846–852.
Article18. Takeshita M, Iwashita A, Kurihara K, Ikejiri K, Higashi H, Udoh T, Kikuchi M. Histologic and immunohistologic findings and prognosis of 40 cases of gastric large B-cell lymphoma. Am J Surg Pathol. 2000. 24:1641–1649.
Article19. Ohshima K, Kawasaki C, Muta H, Muta K, Deyev V, Haraoka S, Suzumiya J, Podack ER, Kikuchi M. CD10 and Bcl10 expression in diffuse large B-cell lymphoma: CD10 is a marker of improved prognosis. Histopathology. 2001. 39:156–162.
Article20. Go JH, Yang WI, Ree HJ. CD10 expression in primary intestinal large B-cell lymphomas: its clinical significance. Arch Pathol Lab Med. 2002. 126:956–960.21. Uherova P, Ross CW, Schnitzer B, Singleton TP, Finn WG. The clinical significance of CD10 antigen expression in diffuse large B-cell lymphoma. Am J Clin Pathol. 2001. 115:582–588.
Article22. Biasoli I, Morais JC, Scheliga A, Milito CB, Romano S, Land M, Pulcheri W, Spector N. CD10 and Bcl-2 expression combined with the International Prognostic Index can identify subgroups of patients with diffuse large-cell lymphoma with very good or very poor prognoses. Histopathology. 2005. 46:328–333.
Article23. Fabiani B, Delmer A, Lepage E, Guettier C, Petrella T, Briere J, Penny AM, Copin MC, Diebold J, Reyes F, Gaulard P, Molina TJ. Groupe d'Etudes des Lymphomes de l'Adulte. CD10 expression in diffuse large B-cell lymphomas does not influence survival. Virchows Arch. 2004. 445:545–551.24. Xu Y, McKenna RW, Kroft SH. Comparison of multiparameter flow cytometry with cluster analysis and immunohistochemistry for the detection of CD10 in diffuse large B-Cell lymphomas. Mod Pathol. 2002. 15:413–419.
Article25. Falini B, Mason DY. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Blood. 2002. 99:409–426.
Article26. Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995. 86:45–53.
Article27. Zhang A, Ohshima K, Sato K, Kanda M, Suzumiya J, Shimazaki K, Kawasaki C, Kikuchi M. Prognostic clinicopathologic factors, including immunologic expression in diffuse large B-cell lymphomas. Pathol Int. 1999. 49:1043–1052.
Article28. Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, de Jong D, Maartense E, Schuuring E, Kluin PM. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998. 92:3152–3162.
Article29. Kawasaki C, Ohshim K, Suzumiya J, Kanda M, Tsuchiya T, Tamura K, Kikuchi M. Rearrangements of bcl-1, bcl-2, bcl-6, and c-myc in diffuse large B-cell lymphomas. Leuk Lymphoma. 2001. 42:1099–1106.
Article30. Falini B, Fizzotti M, Pucciarini A, Bigerna B, Marafioti T, Gambacorta M, Pacini R, Alunni C, Natali-Tanci L, Ugolini B, Sebastiani C, Cattoretti G, Pileri S, Dalla-Favera R, Stein H. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000. 95:2084–2092.
Article31. Tsuboi K, Iida S, Inagaki H, Kato M, Hayami Y, Hanamura I, Miura K, Harada S, Kikuchi M, Komatsu H, Banno S, Wakita A, Nakamura S, Eimoto T, Ueda R. MUM1/IRF4 expression as a frequent event in mature lymphoid malignancies. Leukemia. 2000. 14:449–456.
Article32. Carbone A, Gloghini A, Gaidano G, Franceschi S, Capello D, Drexler HG, Falini B, Dalla-Favera R. Expression status of BCL-6 and syndecan-1 identifies distinct histogenetic subtypes of Hodgkin's disease. Blood. 1998. 92:2220–2228.33. Colomo L, Loong F, Rives S, Pittaluga S, Martinez A, Lopez-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004. 28:736–747.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of p16 in Diffuse Large B-cell Lymphoma and Its Prognostic Implications
- Prognostic Significance of P53, BCL-2 and PCNA in Diffuse Large B-Cell Lymphoma: Correlation with International Prognostic Index
- A Case of Primary Cutaneous Diffuse Large B-cell Lymphoma
- Prognostic Implication of Semi-quantitative Immunohistochemical Assessment of CD20 Expression in Diffuse Large B-Cell Lymphoma
- Relevance of prognostic index with β2-microglobulin for patients with diffuse large B-cell lymphoma in the rituximab era