J Pathol Transl Med.
2016 Mar;50(2):96-103. 10.4132/jptm.2016.01.12.
Prognostic Implication of Semi-quantitative Immunohistochemical Assessment of CD20 Expression in Diffuse Large B-Cell Lymphoma
- Affiliations
-
- 1Department of Pathology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea. sukjinchoi007@gmail.com
- 2Department of Hematology-Oncology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
- KMID: 2211376
- DOI: http://doi.org/10.4132/jptm.2016.01.12
Abstract
- BACKGROUND
Immunohistochemical demonstration of CD20 in diffuse large B-cell lymphoma (DLBCL) is prerequisite not only for the diagnosis but also for assigning patients to rituximab-containing chemotherapy. However, little is known about the impact of abundance of CD20 expression assessed by immunohistochemistry on the clinical outcome of DLBCL. We performed a semi-quantitative immunohistochemical analysis of CD20 expression in DLBCL to examine the prognostic implication of the level of CD20 expression.
METHODS
Pre-treatment diagnostic tissue samples from 48 DLBCL patients who were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen were represented in a tissue microarray and immunostained for CD20. The relative abundance of CD20 expression was semi-quantitatively scored using a web-based ImmunoMembrane plug-in. Receiver operating characteristic curve analysis was used to determine a prognostically relevant cut-off score in order to dichotomize the patients into CD20-high versus CD20-low groups.
RESULTS
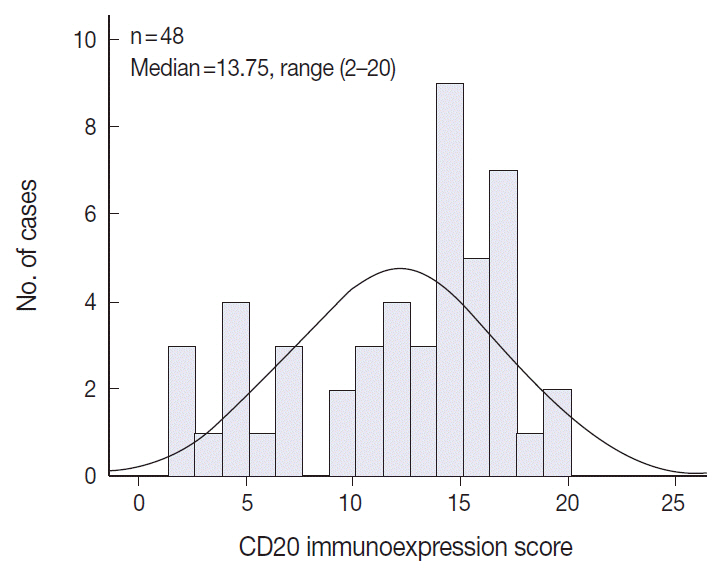
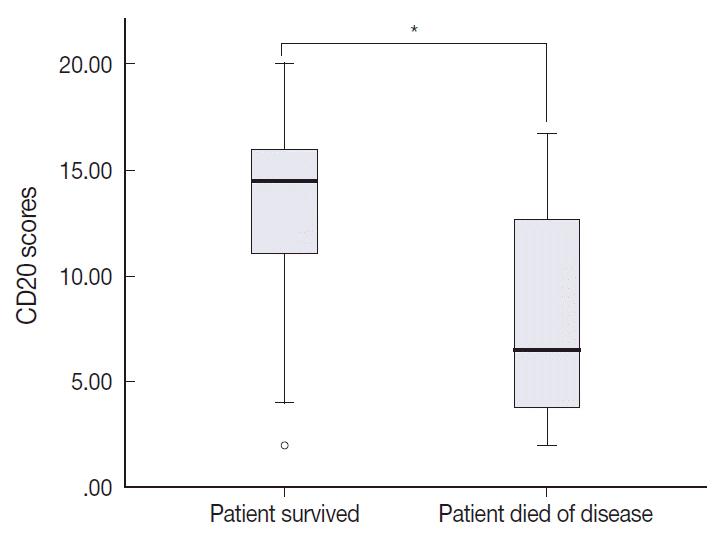
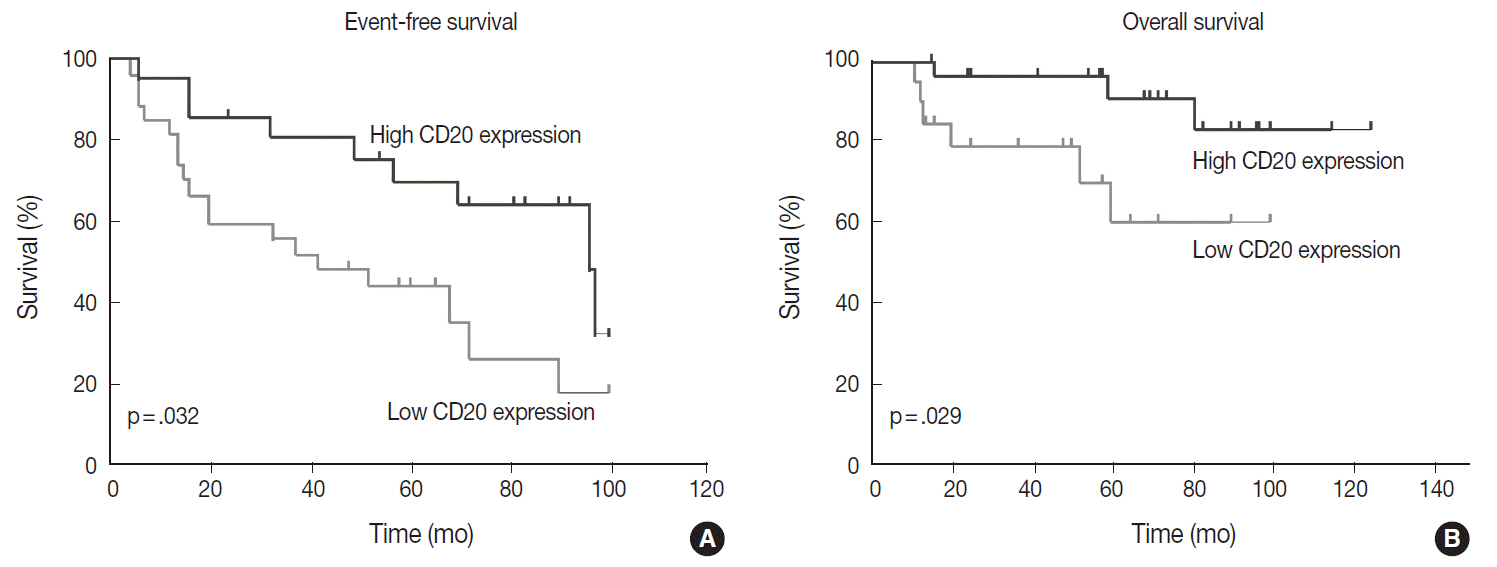
The levels of CD20 expression were heterogeneous among the patients, with a wide and linear distribution of scores. Patients in CD20-low group showed significantly poor clinical outcome.
CONCLUSIONS
The levels of CD20 expression in DLBCL are heterogeneous among the patients with DLBCL. A subgroup of the patients with CD20 expression levels below the cut-off score showed poor clinical outcome.
MeSH Terms
Figure
Reference
-
1. Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008. p. 233–7.2. Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005; 23:6387–93.
Article3. Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012; 2:676–90.4. Kim KH, Choi SJ, Choi YI, et al. In-house manual construction of high-density and high-quality tissue microarrays by using homemade recipient agarose-paraffin blocks. Korean J Pathol. 2013; 47:238–44.
Article5. Berglund M, Thunberg U, Amini RM, et al. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005; 18:1113–20.
Article6. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.
Article7. Tuominen VJ, Tolonen TT, Isola J. ImmunoMembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathology. 2012; 60:758–67.
Article8. Salles G, de Jong D, Xie W, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg Lymphoma Biomarker Consortium. Blood. 2011; 117:7070–8.
Article9. Culpin RE, Sieniawski M, Angus B, et al. Prognostic significance of immunohistochemistry-based markers and algorithms in immunochemotherapy-treated diffuse large B cell lymphoma patients. Histopathology. 2013; 63:788–801.
Article10. Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007; 26:3603–13.
Article11. Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003; 101:4279–84.
Article12. Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007; 109:4930–5.
Article13. Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006; 107:4207–13.
Article14. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007; 109:1857–61.
Article15. Johnson NA, Boyle M, Bashashati A, et al. Diffuse large B-cell lymphoma: reduced CD20 expression is associated with an inferior survival. Blood. 2009; 113:3773–80.
Article16. Olejniczak SH, Stewart CC, Donohue K, Czuczman MS. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest. 2006; 35:93–114.
Article17. Horvat M, Kloboves Prevodnik V, Lavrencak J, Jezersek Novakovic B. Predictive significance of the cut-off value of CD20 expression in patients with B-cell lymphoma. Oncol Rep. 2010; 24:1101–7.
Article18. Suzuki Y, Yoshida T, Wang G, et al. Association of CD20 levels with clinicopathological parameters and its prognostic significance for patients with DLBCL. Ann Hematol. 2012; 91:997–1005.
Article19. Shimizu R, Kikuchi J, Wada T, Ozawa K, Kano Y, Furukawa Y. HDAC inhibitors augment cytotoxic activity of rituximab by upregulating CD20 expression on lymphoma cells. Leukemia. 2010; 24:1760–8.
Article20. Damm JK, Gordon S, Ehinger M, et al. Pharmacologically relevant doses of valproate upregulate CD20 expression in three diffuse large B-cell lymphoma patients in vivo. Exp Hematol Oncol. 2015; 4:4.
Article21. Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008; 111:1094–100.
Article22. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014; 370:1101–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metachronous extranodal natural killer/T-cell lymphoma of nasal type and primary testicular lymphoma
- The Expression of p16 in Diffuse Large B-cell Lymphoma and Its Prognostic Implications
- Frequency of bcl-2 mbr/JH Rearrangement and Prognostic Implication of bcl-2, c-myc Protein Expression in Non-Hodgkin's Lymphoma
- Frequently Relapsing Secondary Cutaneous Diffuse Large B-cell Lymphoma in the Skin
- Prognostic Evaluation of Nodal Diffuse Large B Cell Lymphoma by Immunohistochemical Profiles with Emphasis on CD138 Expression as a Poor Prognostic Factor