J Korean Med Sci.
2010 Mar;25(3):393-398. 10.3346/jkms.2010.25.3.393.
Clinical Features and Outcomes of Idiopathic Pulmonary Alveolar Proteinosis in Korean Population
- Affiliations
-
- 1Division of Pulmonary Medicine, Department of Internal Medicine, Yonsei University, College of Medicine, Yonsei University Health System, Seoul, Korea. pms70@yuhs.ac
- 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine and Lung Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Division of Pulmonary and Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea.
- 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 7Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Hospital, Seoul, Korea.
- 8Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 9Division of Pulmonology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 10Division of Pulmonary, Allergy & Critical Care Medicine, Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 11Pulmonary Division, Department of Internal Medicine, Inha University Hospital, Incheon, Korea.
- KMID: 1778034
- DOI: http://doi.org/10.3346/jkms.2010.25.3.393
Abstract
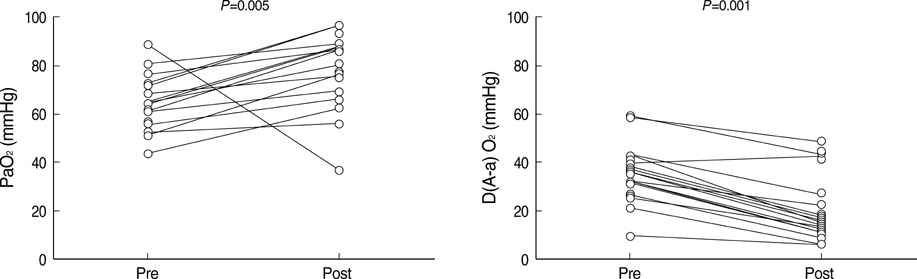
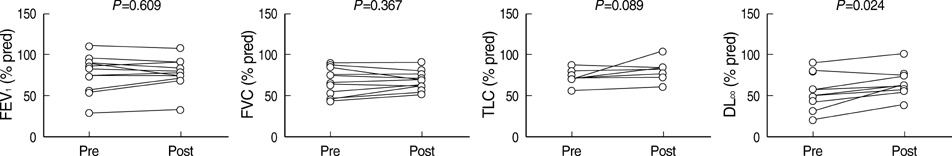
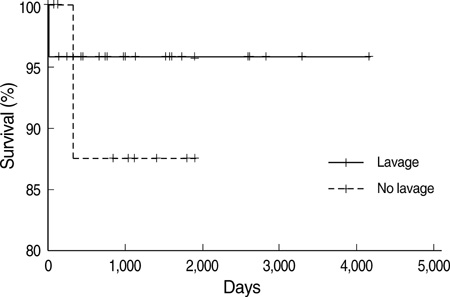
- Idiopathic pulmonary alveolar proteinosis (PAP) is a rare disorder in which lipoproteinaceous material accumulates within alveoli. There were few reports on Asian populations with idiopathic PAP. We retrospectively reviewed 38 patients with idiopathic PAP in Korea. We assessed clinical features, therapeutic efficacy and outcomes of whole lung lavage in patients with idiopathic PAP. The mean age at diagnosis was 52 yr. Eighty six percent of patients were symptomatic at diagnosis. Dyspnea and cough were the most common symptoms. Crackles were the most common physical examination finding. On pulmonary function test, a mild restrictive ventilatory defect was common, with a predicted mean forced vital capacity (FVC) of 77% and forced expiratory volume in one second (FEV1) of 84.6%. Diffusing capacity was disproportionately reduced at 67.7%. Arterial blood gas analysis revealed hypoxemia with a decreased PaO2 of 69.0 mmHg and an increased D(A-a)O2 of 34.2 mmHg. After whole lung lavage, PaO2, D(A-a)O2 and DLCO were significantly improved, but FVC and total lung capacity (TLC) were not different. This is the first multicenter study to analyze 38 Korean patients with idiopathic PAP. The clinical features and pulmonary parameters of Korean patients with idiopathic PAP are consistent with reports in other published studies. Whole lung lavage appears to be the most effective form of treatment.
MeSH Terms
Figure
Cited by 1 articles
-
Pulmonary alveolar proteinosis in a 15-year-old girl
Yechan Kyung, Jihyun Kim, Hong Kwan Kim, Joungho Han, Kangmo Ahn
Allergy Asthma Respir Dis. 2015;3(1):86-89. doi: 10.4168/aard.2015.3.1.86.
Reference
-
1. Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958. 258:1123–1142.
Article2. Teja K, Cooper PH, Squires JE, Schnatterly PT. Pulmonary alveolar proteinosis in four siblings. N Engl J Med. 1981. 305:1390–1392.
Article3. Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993. 328:406–410.4. Nogee LM, Dunbar AE 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001. 344:573–579.
Article5. Kavuru MS, Bonfield TL, Thompson MJ. Mason RJ, Broaddus VC, Murray JF, Nadel JA, editors. Pulmonary alveolar proteinosis. Textbook of pulmonary medicine. 2006. 4th ed. Philadelphia, PA: Elsevier;1716–1734.6. Ben-Dov I, Kishinevski Y, Roznman J, Soliman A, Bishara H, Zelligson E, Grief J, Mazar A, Perelman M, Vishnizer R, Weiler-Ravel D. Pulmonary alveolar proteinosis in Israel: ethnic clustering. Isr Med Assoc J. 1999. 1:75–78.7. deMello DE, Lin Z. Pulmonary alveolar proteinosis: a review. Pediatr Pathol Mol Med. 2001. 20:413–432.
Article8. Goldstein LS, Kavuru MS, Curtis-McCarthy P, Christie HA, Farver C, Stoller JK. Pulmonary alveolar proteinosis: clinical features and outcomes. Chest. 1998. 114:1357–1362.9. Prakash UB, Barham SS, Carpenter HA, Dines DE, Marsh HM. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987. 62:499–518.
Article10. Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002. 166:215–235.11. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994. 264:713–716.
Article12. Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994. 91:5592–5596.
Article13. Seymour JF, Dunn AR, Vincent JM, Presneill JJ, Pain MC. Efficacy of granulocyte-macrophage colony-stimulating factor in acquired alveolar proteinosis. N Engl J Med. 1996. 335:1924–1925.
Article14. Bonfield TL, Kavuru MS, Thomassen MJ. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol. 2002. 105:342–350.
Article15. Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, Vincent JM, Nakata K, Kitamura T, Langton D, Pain MC, Dunn AR. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic idiopathic alveolar proteinosis. Am J Respir Crit Care Med. 2001. 163:524–531.16. Tazawa R, Hamano E, Arai T, Ohta H, Ishimoto O, Uchida K, Watanabe M, Saito J, Takeshita M, Hirabayashi Y, Ishige I, Eishi Y, Hagiwara K, Ebina M, Inoue Y, Nakata K, Nukiwa T. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005. 171:1142–1149.
Article17. Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003. 349:2527–2539.
Article18. Carraway MS, Ghio AJ, Carter JD, Piantadosi CA. Detection of granulocyte-macrophage colony-stimulating factor in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000. 161:1294–1299.
Article19. Kitamura T, Tanaka N, Watanabe J, Uchida , Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999. 190:875–880.
Article20. Asamoto H, Kitaichi M, Nishimura K, Itoh H, Izumi T. Primary pulmonary alveolar proteinosis: clinical observations of 68 patients in Japan. Nihon Kyobu Shikkan Gakkai Zasshi. 1995. 33:835–845.21. Kim HT, Chung HS, Han SK, Shim Y, Kim KY, Han YC. A case of pulmonary alveolar proteinosis. Korean J Intern Med. 1987. 33:668–674.22. Kim G, Lee SJ, Lee HP, Yoo CG, Han SK, Shim YS, Kim YW. The clinical characteristics of pulmonary alveolar proteinosis: experience at Seoul National University Hospital, and review of the literature. J Korean Med Sci. 1999. 14:159–164.
Article23. Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008. 177:752–762.
Article24. Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax. 2000. 55:67–77.25. Wang BM, Stern EJ, Schmidt RA, Pierson DJ. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest. 1997. 111:460–466.26. Iyonaga K, Suga M, Yamamoto T, Ichiyasu H, Miyakawa H, Ando M. Elevated bronchoalveolar concentrations of MCP-1 in patients with pulmonary alveolar proteinosis. Eur Respir J. 1999. 14:383–389.
Article27. Schoch OD, Schanz U, Koller M, Nakata K, Seymour JF, Russi EW, Boehler A. BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax. 2002. 57:277–280.
Article28. Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung. 1984. 162:223–231.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anesthetic Management for Whole-Lung Lavage in a Patient with Pulmonary Alveolar Proteinosis
- A case of Idiopathic CD4+ T-Lymphocytopenia with disseminated Mycobacterium kansasii infection and Pulmonary alveolar proteinosis
- Bilateral Sequential Bronchopulmonary Lavage in One Stage for Recurred Pulmonary Alveolar Proteinosis: A case report
- Anesthetic Management of Lung Lavage in Patient with Pulmonary Alveolar Proteinosis Related to Pneumoconiosis: A case report
- Anesthetic Management for Sequential Bronchoalveolar Lavage in a Patient with Pulmonary Alveolar Proteinosis: A case report