Prescription Pattern of NSAIDs and the Prevalence of NSAID-induced Gastrointestinal Risk Factors of Orthopaedic Patients in Clinical Practice in Korea
- Affiliations
-
- 1Department of Orthopaedic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hacw@skku.edu
- 2Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1777888
- DOI: http://doi.org/10.3346/jkms.2011.26.4.561
Abstract
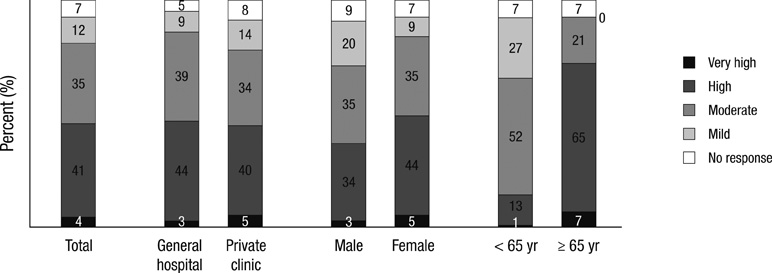
- This is a cross-sectional observational study undertaken to explore the current prescription pattern of non-steroidal anti-inflammatory drugs (NSAIDs) and the prevalence of NSAID-induced gastrointestinal (GI) risk factors of orthopaedic patients in real clinical practice in Korea. Study cohort included 3,140 orthopaedic outpatients at 131 hospitals and clinics between January 2008 and August 2008. A self-administered questionnaire was completed by each patient and physician. A simplified risk scoring scale (the Standardized Calculator of Risk for Events; SCORE) was used to measure patients' risk for GI complications. The pattern of NSAIDs prescription was identified from medical recordings. Forty-five percents of the patients belonged to high risk or very high risk groups for GI complications. The cyclooxygenase-2 enzyme (COX-2) selective NSAID showed a propensity to be prescribed more commonly for high/very high GI risk groups, but the rate was still as low as 51%. In conclusion, physician's considerate prescription of NSAIDs with well-understanding of each patient's GI risk factors is strongly encouraged in order to maximize cost effectiveness and to prevent serious GI complications in Korea. Other strategic efforts such as medical association-led education programs and application of Korean electronic SCORE system to hospital order communication system (OCS) should also be accompanied in a way to promote physician's attention.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Anti-Inflammatory Agents, Non-Steroidal/*adverse effects
Cohort Studies
Cross-Sectional Studies
Cyclooxygenase 2/metabolism
Cyclooxygenase 2 Inhibitors/adverse effects
Drug Prescriptions
Female
Gastrointestinal Diseases/chemically induced/complications/*epidemiology
Humans
Male
Middle Aged
Musculoskeletal Diseases/complications/*drug therapy
Prevalence
Questionnaires
Republic of Korea
Risk Factors
Figure
Cited by 3 articles
-
Current Guidelines for Non-Steroidal Anti-Inflammatory Drugs
Min-Gyue Park, Jae-Doo Yoo, Kyu-Ho Lee
J Korean Orthop Assoc. 2020;55(1):9-28. doi: 10.4055/jkoa.2020.55.1.9.Gastrointestinal Risk Factors and Non-steroidal Anti-inflammatory Drugs Use in Rheumatoid Arthritis and Osteoarthritis Patients in Korea
Eun Young Lee, Seung-Jae Hong, Yong-Beom Park, Kyung-Su Park, Chan-Bum Choi, Chang-Keun Lee, Ran Song, Yun Jong Lee, Chang Hee Suh, Hyun Ah Kim, Jun Ki Min, Chong-Hyeon Yoon, Won Park, Won Tae Chung, Geun-Tae Kim, Jung-Yoon Choe, Seong Wook Kang, Yong-Wook Park, Wan-Hee Yoo, Sang-Heon Lee
J Rheum Dis. 2016;23(1):47-54. doi: 10.4078/jrd.2016.23.1.47.Gastrointestinal Risk Assessment in the Patients Taking Nonsteroidal Anti-inflammarory Drugs for Lumbar Spinal Disease
Byung ho Lee, Byung-Joon Shin, Dong Jun Kim, Jae Chul Lee, Kyung Soo Suk, Ye-Soo Park, Ki-Won Kim, Kyu Jung Cho, Keun-young Shin, Min-suk Koh, Seong-Hwan Moon
J Korean Soc Spine Surg. 2011;18(4):239-245. doi: 10.4184/jkss.2011.18.4.239.
Reference
-
1. Schlansky B, Hwang JH. Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy. J Gastroenterol. 2009. 44:Suppl 19. 44–52.2. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991. 91:213–222.3. Johnson RE, Hornbrook MC, Hooker RS, Woodson GT, Shneidman R. Analysis of the costs of NSAID-associated gastropathy. Experience in a US health maintenance organisation. Pharmacoeconomics. 1997. 12:76–88.4. Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective--1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl. 1998. 51:8–16.5. White TJ, Arakelian A, Rho JP. Counting the costs of drug-related adverse events. Pharmacoeconomics. 1999. 15:445–458.6. Tramèr MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000. 85:169–182.7. Singh G, Triadafilopoulos G. Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl. 1999. 56:18–24.8. Fries JF. NSAID GI toxicity: epidemiology. J Musculoskel Med. 1991. 8:21–28.9. Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991. 114:735–740.10. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med. 1993. 153:1665–1670.11. Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991. 115:787–796.12. Gutthann SP, García Rodríguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997. 8:18–24.13. Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995. 123:241–249.14. McIntosh JH, Fung CS, Berry G, Piper DW. Smoking, nonsteroidal anti-inflammatory drugs, and acetaminophen in gastric ulcer. A study of associations and of the effects of previous diagnosis on exposure patterns. Am J Epidemiol. 1988. 128:761–770.15. Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998. 105:31S–38S.16. Bull SA, Conell C, Campen DH. Relationship of clinical factors to the use of COX-2 selective NSAIDs within an arthritis population in a large HMO. J Manag Care Pharm. 2002. 8:252–258.17. García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007. 132:498–506.18. Koncz TA, Lister SP, Makinson GT. Gastroprotection in patients prescribed non-selective NSAIDs, and the risk of related hospitalization. Curr Med Res Opin. 2008. 24:3405–3412.19. Taha AS, Dahill S, Sturrock RD, Lee FD, Russell RI. Predicting NSAID related ulcers--assessment of clinical and pathological risk factors and importance of differences in NSAID. Gut. 1994. 35:891–895.20. Fries JF, Bruce B. Rates of serious gastrointestinal events from low dose use of acetylsalicylic acid, acetaminophen, and ibuprofen in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003. 30:2226–2233.21. Cheetham TC, Levy G, Spence M. Predicting the risk of gastrointestinal bleeding due to nonsteroidal antiinflammatory drugs: NSAID electronic assessment of risk. J Rheumatol. 2003. 30:2241–2244.22. NSAID-induced GI management study. MO online. c2000-2011. accessed on14 Mar 2011. Korea: Medical observer;Available at http://www.moonline.co.kr/News/news_view.aspx?Cid=H1707&Cno=38293.23. Coté GA, Rice JP, Bulsiewicz W, Norvell JP, Christensen K, Bobb A, Postelnick M, Howden CW. Use of physician education and computer alert to improve targeted use of gastroprotection among NSAID users. Am J Gastroenterol. 2008. 103:1097–1103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NSAID-induced Gastroenteropathy
- Prevention of Non-steroidal Anti-inflammatory Drug-induced Peptic Ulcers
- Gastrointestinal Risk Assessment in the Patients Taking Nonsteroidal Anti-inflammarory Drugs for Lumbar Spinal Disease
- The Pattern of Use of Oral NSAIDs with or without Co-prescription of Gastroprotective Agent for Arthritic Knee by Korean Practitioners
- Risk of Lower Gastrointestinal Bleeding in Nonsteroidal Anti-inflammatory Drug (NSAID) and Proton Pump Inhibitor Users Compared with NSAID-Only Users: A Common Data Model Analysis