J Korean Med Sci.
2013 Nov;28(11):1682-1686. 10.3346/jkms.2013.28.11.1682.
An Unusual Presentation of Diabetic Ketoacidosis in Familial Hajdu-Cheney Syndrome: A Case Report
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea. yschung@ajou.ac.kr
- 2Department of Medical Genetics, Ajou University School of Medicine, Suwon, Korea.
- KMID: 1777670
- DOI: http://doi.org/10.3346/jkms.2013.28.11.1682
Abstract
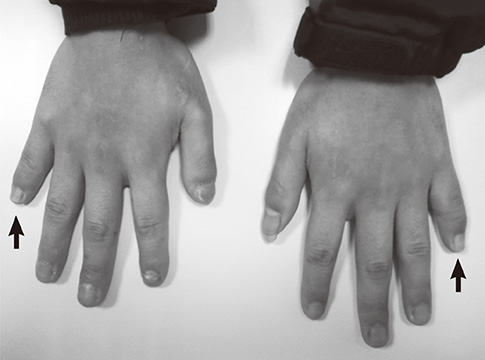
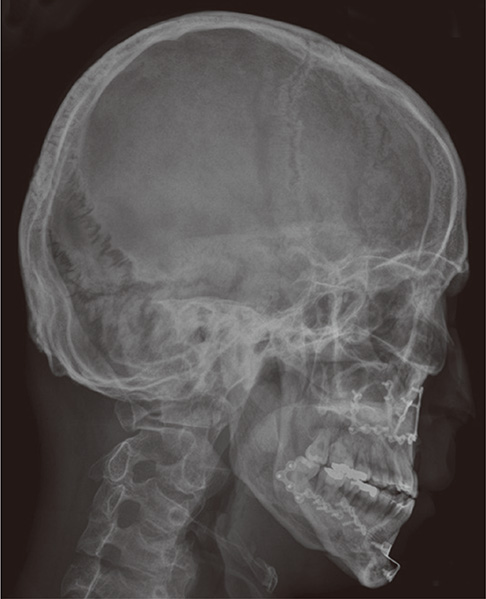
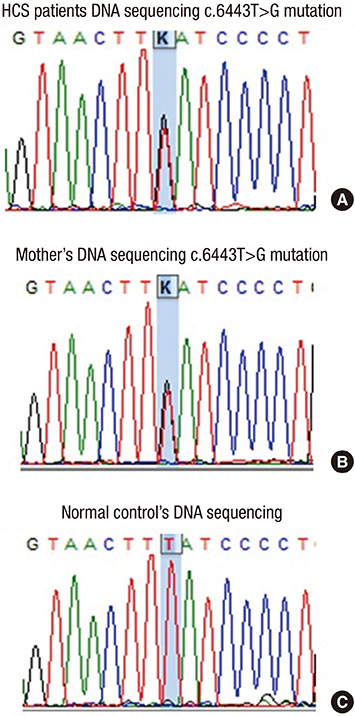
- A 21-year-old man with diabetic ketoacidosis (DKA) displayed short and clubbed fingers and marked eyebrow, which are typical of Hajdu-Cheney Syndrome (HCS). Laboratory findings confirmed type 1 diabetes mellitus (DM). After conservative care with hydration and insulin supply, metabolic impairment was improved. Examinations of bone and metabolism revealed osteoporosis and craniofacial abnormalities. The mutation (c.6443T>G) of the NOTCH2 gene was found. The patient was diagnosed with HCS and DM. There may be a relationship between HCS and DM, with development of pancreatic symptoms related to the NOTCH2 gene mutation.
MeSH Terms
-
Adult
Bone Density
Craniofacial Abnormalities/complications/radiography
Diabetes Mellitus, Type 1/*complications/diagnosis
Diabetic Ketoacidosis/complications/genetics
Glycosuria
Hajdu-Cheney Syndrome/*complications/diagnosis/radiography
Humans
Ketone Bodies/urine
Male
Mutation
Osteoporosis/complications/radiography
Receptor, Notch2/*genetics
Young Adult
Ketone Bodies
Receptor, Notch2
Figure
Reference
-
1. Currarino G. Hajdu-Cheney syndrome associated with serpentine fibulae and polycystic kidney disease. Pediatr Radiol. 2009; 39:47–52.2. Hajdu N, Kauntze R. Cranio-skeletal dysplasia. Br J Radiol. 1948; 21:42–48.3. Cheney WD. Acro-osteolysis. Am J Roentgenol Radium Ther Nucl Med. 1965; 94:595–607.4. Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011; 43:303–305.5. Ornetti P, Tavernier C. Osteoporotic compression fracture revealing Hajdu-Cheney syndrome. Joint Bone Spine. 2012; 79:514–515.6. Stathopoulos IP, Trovas G, Lampropoulou-Adamidou K, Koromila T, Kollia P, Papaioannou NA, Lyritis G. Severe osteoporosis and mutation in NOTCH2 gene in a woman with Hajdu-Cheney syndrome. Bone. 2013; 52:366–371.7. Han EJ, Mun JI, An SY, Jung YJ, Kim OH, Chung YS. A case report of Hajdu-Cheney syndrome. Endocrinol Metab. 2010; 25:152–156.8. Narumi Y, Min BJ, Shimizu K, Kazukawa I, Sameshima K, Nakamura K, Kosho T, Rhee Y, Chung YS, Kim OH, et al. Clinical consequences in truncating mutations in exon 34 of NOTCH2: report of six patients with Hajdu-Cheney syndrome and a patient with serpentine fibula polycystic kidney syndrome. Am J Med Genet A. 2013; 161A:518–526.9. Brennan AM, Pauli RM. Hajdu-Cheney syndrome: evolution of phenotype and clinical problems. Am J Med Genet. 2001; 100:292–310.10. Zanotti S, Canalis E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif Tissue Int. 2012; 90:69–75.11. Kung AW, Xiao SM, Cherny S, Li GH, Gao Y, Tso G, Lau KS, Luk KD, Liu JM, Cui B, et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am J Hum Genet. 2010; 86:229–239.12. McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006; 79:169–173.13. Majewski J, Schwartzentruber JA, Caqueret A, Patry L, Marcadier J, Fryns JP, Boycott KM, Ste-Marie LG, McKiernan FE, Marik I, et al. FORGE Canada Consortium. Mutations in NOTCH2 in families with Hajdu-Cheney syndrome. Hum Mutat. 2011; 32:1114–1117.14. Ninov N, Borius M, Stainier DY. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development. 2012; 139:1557–1567.15. Lammert E, Brown J, Melton DA. Notch gene expression during pancreatic organogenesis. Mech Dev. 2000; 94:199–203.16. Li HJ, Kapoor A, Giel-Moloney M, Rindi G, Leiter AB. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev Biol. 2012; 371:156–169.17. Afelik S, Qu X, Hasrouni E, Bukys MA, Deering T, Nieuwoudt S, Rogers W, Macdonald RJ, Jensen J. Notch-mediated patterning and cell fate allocation of pancreatic progenitor cells. Development. 2012; 139:1744–1753.18. Bar Y, Russ HA, Sintov E, Anker-Kitai L, Knoller S, Efrat S. Redifferentiation of expanded human pancreatic β-cell-derived cells by inhibition of the NOTCH pathway. J Biol Chem. 2012; 287:17269–17280.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Zoledronic Acid on Acro-Osteolysis and Osteoporosis in a Patient with Hajdu-Cheney Syndrome
- A Case Report of Hajdu-Cheney Syndrome
- Insulin Autoimmune Syndrome with Diabetic Ketoacidosis
- New Onset Diabetic Ketoacidosis Associated with Quetiapine
- A Case of Poland Syndrome with Diabetic Ketoacidosis