Yonsei Med J.
2012 May;53(3):625-633. 10.3349/ymj.2012.53.3.625.
Altered Cellular Kinetics in the Growth Plate of the Femoral Head of Spontaneously Hypertensive Rats
- Affiliations
-
- 1Department of Orthopaedic Surgery, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. pedhkim@yuhs.ac
- KMID: 1777000
- DOI: http://doi.org/10.3349/ymj.2012.53.3.625
Abstract
- PURPOSE
Pathologic changes in the growth plate remain unknown in Legg-Calve-Perthes (LCP) disease. Spontaneously hypertensive rats have proven to be a good model for studying LCP disease. This study investigated the histopathologic changes and the expression of vascular endothelial growth factor in the growth plate of spontaneously hypertensive rats (SHR).
MATERIALS AND METHODS
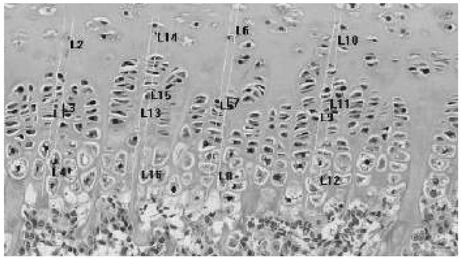
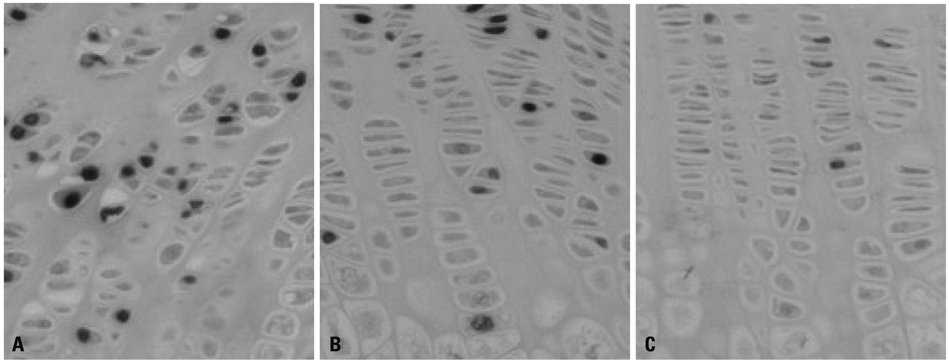
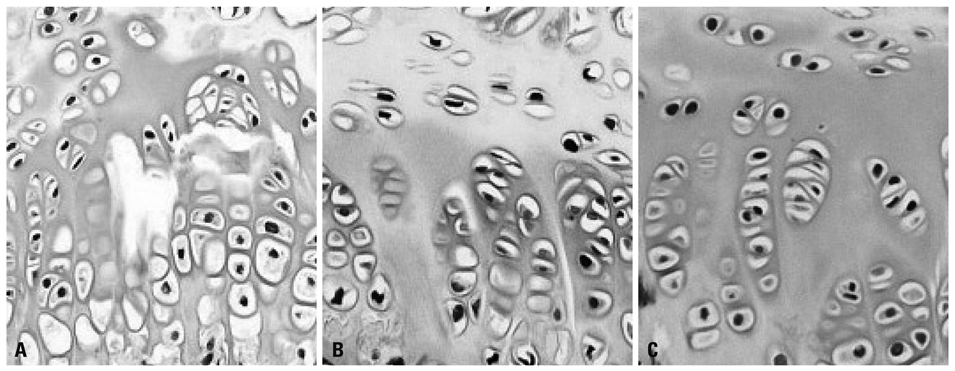
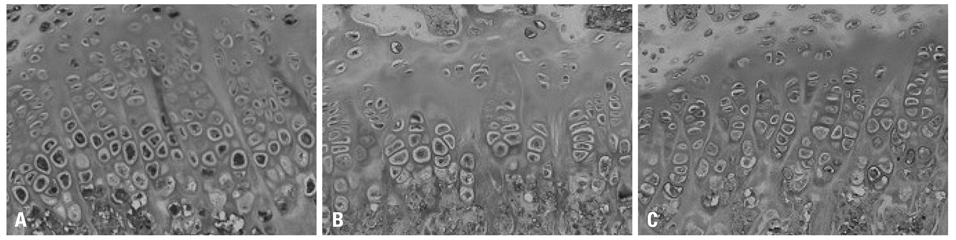
Sixty SHR rats were divided into two groups: those showing osteonecrosis (SHR+n group: 32), and those showing normal ossification (SHR-n group: 28). Thirty Wister Kyoto rats served as a control. For histomorphological measurement, the length of each zone of the growth plate was measured. Cell kinetics was measured by 5-bromo-2'-deoxyuridin (BrdU) immunohistochemistry and transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) assays. Vascular endothelial growth factor (VEGF) immunohistochemistry was used to identify of expression of VEGF.
RESULTS
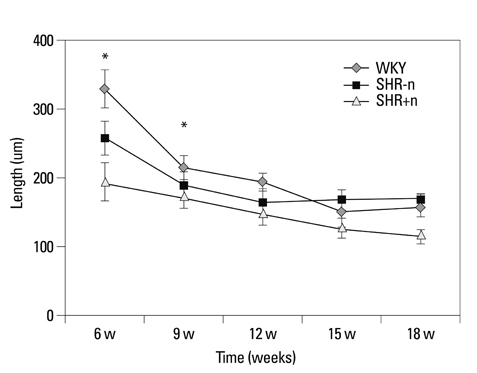
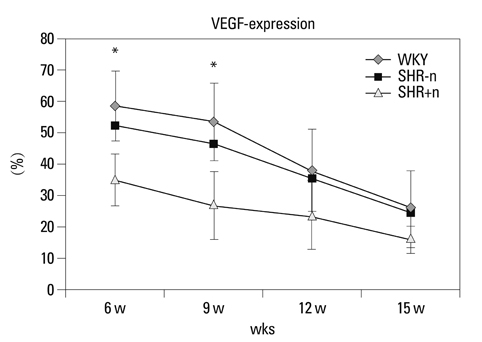
The lengths of growth plates of the SHR+n group were significantly shorter in the initial growth period than those of the other groups. The lowest proliferative rate and the highest apoptosis rate were observed in the SHR+n group at the initial growth period. The expression of VEGF in the growth plate of the SHR group was lower than the control group, and it was lower in the SHR+n group than in the SHR-n group.
CONCLUSION
The growth plate of the SHR+n group was found to be affected by disease process of ischemic necrosis of the femoral head, and this might explain the relative overgrowth of the greater trochanter in the later stages of LCP disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Ponseti IV, Maynard JA, Weinstein SL, Ippolito EG, Pous JG. Legg-Calvé-Perthes disease. Histochemical and ultrastructural observations of the epiphyseal cartilage and physis. J Bone Joint Surg Am. 1983. 65:797–807.
Article2. Schoenecker PL, Stone JW, Capelli AM. Legg-Perthes disease in children under 6 years old. Orthop Rev. 1993. 22:201–208.
Article3. Oda J, Hirano T, Iwasaki K, Majima R. Vascular occlusion and cartilage disorders in osteonecrosis of the femoral head in rats. Int Orthop. 1996. 20:185–189.
Article4. Kim HT, Wenger DR. "Functional retroversion" of the femoral head in Legg-Calvé-Perthes disease and epiphyseal dysplasia: analysis of head-neck deformity and its effect on limb position using three-dimensional computed tomography. J Pediatr Orthop. 1997. 17:240–246.
Article5. Zenmyo M, Komiya S, Kawabata R, Sasaguri Y, Inoue A, Morimatsu M. Morphological and biochemical evidence for apoptosis in the terminal hypertrophic chondrocytes of the growth plate. J Pathol. 1996. 180:430–433.
Article6. Kong SY, Kim HW, Park HW, Lee SY, Lee KS. Effects of multiple drilling on the ischemic capital femoral epiphysis of immature piglets. Yonsei Med J. 2011. 52:809–817.
Article7. Kim HK, Su PH, Qiu YS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis. An experimental investigation in immature pigs. J Bone Joint Surg Am. 2001. 83-A:688–697.8. Little DG, McDonald M, Sharpe IT, Peat R, Williams P, McEvoy T. Zoledronic acid improves femoral head sphericity in a rat model of perthes disease. J Orthop Res. 2005. 23:862–868.
Article9. Hirano T, Iwasaki K, Oda J, Kumashiro T. Osteonecrosis of the femoral head in spontaneously hypertensive rats. Relation to ossific nuclei during growth. Acta Orthop Scand. 1992. 63:37–40.10. Naito S, Ito M, Sekine I, Ito M, Hirano T, Iwasaki K, et al. Femoral head necrosis and osteopenia in stroke-prone spontaneously hypertensive rats (SHRSPs). Bone. 1993. 14:745–753.
Article11. Kawahara T, Shimokawa I, Tomita M, Hirano T, Shindo H. Effects of caloric restriction on development of the proximal growth plate and metaphysis of the caput femoris in spontaneously hypertensive rats: microscopic and computer-assisted image analyses. Microsc Res Tech. 2002. 59:306–312.
Article12. Hirano T, Majima R, Yoshida G, Iwasaki K. Characteristics of blood vessels feeding the femoral head liable to osteonecrosis in spontaneously hypertensive rats. Calcif Tissue Int. 1996. 58:201–205.
Article13. Tomita M, Shimokawa I, Maeda H, Higami Y, Kawahara T, Ikeda T, et al. Dietary restriction reduces the prevalence of osteonecrosis of the caput femoris in spontaneously hypertensive rats. Calcif Tissue Int. 1999. 64:259–262.
Article14. Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002. 111:61–73.
Article15. Farnum CE, Wilsman NJ. Determination of proliferative characteristics of growth plate chondrocytes by labeling with bromodeoxyuridine. Calcif Tissue Int. 1993. 52:110–119.
Article16. Wilsman NJ, Farnum CE, Green EM, Lieferman EM, Clayton MK. Cell cycle analysis of proliferative zone chondrocytes in growth plates elongating at different rates. J Orthop Res. 1996. 14:562–572.
Article17. Galotto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res. 1994. 9:1239–1249.
Article18. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999. 5:623–628.
Article19. Matsuno T, Ishida O, Arihiro K, Sunagawa T, Mori N, Ikuta Y. Cell proliferation and death of growth plate chondrocyte caused by ischemia and reperfusion. Microsurgery. 2001. 21:30–36.
Article20. Roach HI, Clarke NM. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br. 2000. 82:601–613.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered Cellular Kinetics in Growth Plate according to Alterations in Weight Bearing
- Expression of Vascular Endothelial Growth Factor in an Animal Model of Avascular Necrosis of the Femoral Head
- Altered Cellular Kinetics in the Growth Plate according to Alterations in the Weight Bearing
- Effect of Cephalosporins on Growth Plates of Femoral Heads of Rats
- Effects of Changes in Mechanical Loading on Endochondral Bone Formation in Hindlimb-suspended Rats