Yonsei Med J.
2012 May;53(3):618-624. 10.3349/ymj.2012.53.3.618.
Altered Cellular Kinetics in Growth Plate according to Alterations in Weight Bearing
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. ihyang@yuhs.ac
- KMID: 1776999
- DOI: http://doi.org/10.3349/ymj.2012.53.3.618
Abstract
- PURPOSE
To examine the effects of change in weight bearing on the growth plate metabolism, a simulated animal model of weightlessness was introduced and the chondrocytes' cellular kinetics was evaluated.
MATERIALS AND METHODS
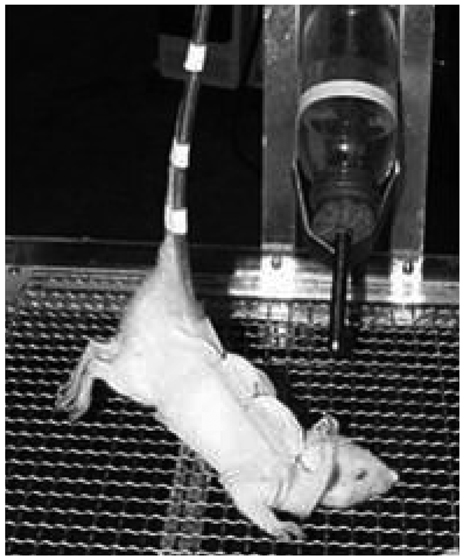
Unloading condition on the hind-limb of Sprague-Dawley rats was created by fixing a tail and lifting the hind-limb. Six rats aged 6 weeks old were assigned to each group of unloading, reloading, and control groups of unloading or reloading. Unloading was maintained for three weeks, and then reloading was applied for another one week thereafter. Histomorphometry for the assessment of vertical length of the growth plate, 5-bromo-2'-deoxyuridin immunohistochemistry for cellular kinetics, and biotin nick end labeling transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) assay for chondrocytes apoptosis in the growth plate were performed.
RESULTS
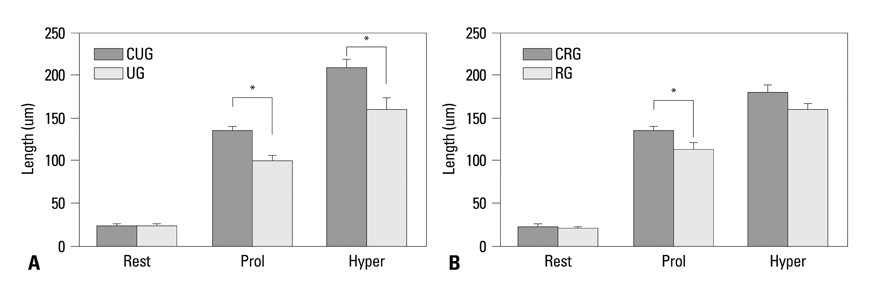
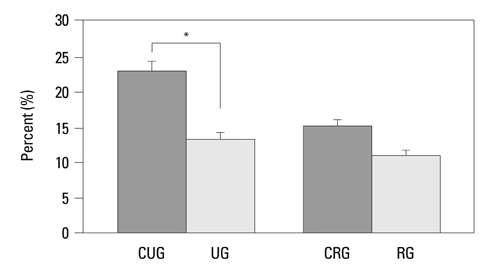
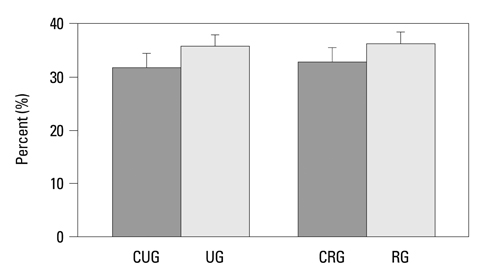
The vertical length of the growth plate and the proliferative potential of chondrocytes were decreased in the unloading group compared to those of control groups. Inter-group differences were more significant in the proliferative and hypertrophic zones. Reloading increased the length of growth plate and proliferative potential of chondrocytes. However, apoptotic changes in the growth plate were not affected by the alterations of weight bearing.
CONCLUSION
Alterations in the weight bearing induced changes in the chondrocytic proliferative potential of the growth plate, however, had no effects on the apoptosis. This may explain why non-weight bearing in various clinical situations hampers normal longitudinal bone growth. Further studies on the factors for reversibility of chondrocytic proliferation upon variable mechanical stresses are needed.
MeSH Terms
Figure
Reference
-
1. Globus RK, Bikle DD, Morey-Holton E. Effects of simulated weightlessness on bone mineral metabolism. Endocrinology. 1984. 114:2264–2270.
Article2. Matsumoto T, Nakayama K, Kodama Y, Fuse H, Nakamura T, Fukumoto S. Effect of mechanical unloading and reloading on periosteal bone formation and gene expression in tail-suspended rapidly growing rats. Bone. 1998. 22:5 Suppl. 89S–93S.
Article3. Nyhan D, Kim S, Dunbar S, Li D, Shoukas A, Berkowitz DE. Impaired pulmonary artery contractile responses in a rat model of microgravity: role of nitric oxide. J Appl Physiol. 2002. 92:33–40.
Article4. Vico L, Lafage-Proust MH, Alexandre C. Effects of gravitational changes on the bone system in vitro and in vivo. Bone. 1998. 22:5 Suppl. 95S–100S.
Article5. Mayr W, Bijak M, Girsch W, Hofer C, Lanmüller H, Rafolt D, et al. MYOSTIM-FES to prevent muscle atrophy in microgravity and bed rest: preliminary report. Artif Organs. 1999. 23:428–431.
Article6. de Rooij PP, Siebrecht MA, Tägil M, Aspenberg P. The fate of mechanically induced cartilage in an unloaded environment. J Biomech. 2001. 34:961–966.
Article7. Gardner TN, Mishra S. The biomechanical environment of a bone fracture and its influence upon the morphology of healing. Med Eng Phys. 2003. 25:455–464.
Article8. Kong SY, Kim HW, Park HW, Lee SY, Lee KS. Effects of multiple drilling on the ischemic capital femoral epiphysis of immature piglets. Yonsei Med J. 2011. 52:809–817.
Article9. Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002. 92:1367–1377.
Article10. Bloomfield SA, Allen MR, Hogan HA, Delp MD. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone. 2002. 31:149–157.
Article11. Streeter GL. Developmental horizons in human embryos; a review of the histogenesis of cartilage and bone. Contrib Embryol. 1949. 33:149–168.12. Chung UI. Essential role of hypertrophic chondrocytes in endochondral bone development. Endocr J. 2004. 51:19–24.
Article13. Wilsman NJ, Farnum CE, Green EM, Lieferman EM, Clayton MK. Cell cycle analysis of proliferative zone chondrocytes in growth plates elongating at different rates. J Orthop Res. 1996. 14:562–572.
Article14. Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000. 10:223–228.
Article15. Karsenty G. Chondrogenesis just ain't what it used to be. J Clin Invest. 2001. 107:405–407.
Article16. Matsuno T, Ishida O, Arihiro K, Sunagawa T, Mori N, Ikuta Y. Cell proliferation and death of growth plate chondrocyte caused by ischemia and reperfusion. Microsurgery. 2001. 21:30–36.
Article17. Duke PJ, Durnova G, Montufar-Solis D. Histomorphometric and electron microscopic analyses of tibial epiphyseal plates from Cosmos 1887 rats. FASEB J. 1990. 4:41–46.
Article18. Sibonga JD, Zhang M, Evans GL, Westerlind KC, Cavolina JM, Morey-Holton E, et al. Effects of spaceflight and simulated weightlessness on longitudinal bone growth. Bone. 2000. 27:535–540.
Article19. Shimano MM, Volpon JB. Biomechanics and structural adaptations of the rat femur after hindlimb suspension and treadmill running. Braz J Med Biol Res. 2009. 42:330–338.
Article20. Wronski TJ, Morey ER. Recovery of the rat skeleton from the adverse effects of simulated weightlessness. Metab Bone Dis Relat Res. 1983. 4:347–352.21. Farnum CE, Wilsman NJ. Determination of proliferative characteristics of growth plate chondrocytes by labeling with bromodeoxyuridine. Calcif Tissue Int. 1993. 52:110–119.
Article22. Vanky P, Brockstedt U, Hjerpe A, Wikström B. Kinetic studies on epiphyseal growth cartilage in the normal mouse. Bone. 1998. 22:331–339.
Article23. Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995. 131:483–494.
Article24. Hatori M, Klatte KJ, Teixeira CC, Shapiro IM. End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J Bone Miner Res. 1995. 10:1960–1968.
Article25. Basso N, Heersche JN. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone. 2006. 39:807–814.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered Cellular Kinetics in the Growth Plate according to Alterations in the Weight Bearing
- Effects of Changes in Mechanical Loading on Endochondral Bone Formation in Hindlimb-suspended Rats
- Effects of Heat Shock and Antioxidant on the Growth Plate of Unloaded Rats
- Altered Cellular Kinetics in the Growth Plate of the Femoral Head of Spontaneously Hypertensive Rats
- A Comparison of Radiographs of the Hallux Valgus and Normal Foot in Women over Forty Years Old on Weight-bearing and Wonweight-bearing